 I. Next-Generation Chemical Probe Engineering
I. Next-Generation Chemical Probe Engineering
RNA-targeted small molecules will transition from “undruggable” challenges to precision therapeutics through:
- Rational Design Platforms
- Machine learning-driven virtual screening of R-BIND database (116 validated RNA ligands) to predict binding pockets in non-coding RNAs
- Dynamic combinatorial chemistry for targeting structurally dynamic RNA elements
(Fig. 1: AI-optimized probe design)
Description: Computational model predicting small molecule (blue) binding to riboswitch pocket (gold) with hydrogen bond networks (dashed lines).
- Epitranscriptomic Probes
- m⁶A-specific chemical probes achieving single-base resolution in live cells
- Selenophene-modified nucleotides enabling cryo-EM structural mapping of RNA-drug complexes

II. Revolutionary Imaging Technologies
A. Live-Cell Imaging Breakthroughs
Technology Mechanism Resolution Gain Frankenbody Probes Engineered antibody-HA tag system 5x faster fluorescence kinetics vs GFP Fluorescent RNA Aptamers Broccoli/Spinach2 tags without transfection Single-transcript tracking Photocaged Systems 405 nm-activatable probes Spatiotemporal control within 2 μm B. Integrated Multi-Omics Visualization
- Cryo-EM Correlative Mapping:
- Sub-3Å resolution of RNA-protein complexes during translation
- Single-Cell Spatial Transcriptomics 2.0:
- RNAscope® combined with proteomics for whole-cell interactome mapping
(Fig. 2: Frankenbody-RNA imaging workflow)
Description: Live neuron showing β-actin mRNA (green) tracked via HA-tagged frankenbody probes with real-time translation sites (red sparks).
III. Clinical Translation Accelerators
A. Theranostic Nanoplatforms
- RNA Origami Systems:
- Self-assembling nanostructures simultaneously capturing oncogenic miRNAs and releasing ASO therapeutics
- CRISPR-Cas13 Integration:
- Collateral cleavage-activated probes for viral load quantification in 20 minutes
B. Automated Diagnostic Ecosystems
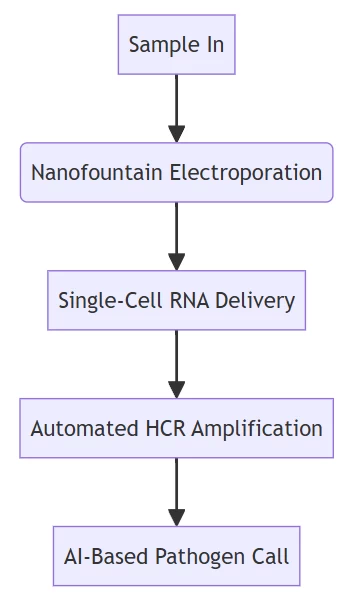
NFP-E technology achieves >95% transfection efficiency with <5% cell mortality
IV. Market Expansion and Commercialization
Projected Growth (2024-2030)
Sector CAGR Driving Technology Cancer Diagnostics 24.7% RNAscope® automation Point-of-Care Testing 31.2% CRISPR-SmartProbes CNS Disorder Therapeutics 28.5% Blood-brain barrier penetrating probes Key Commercial Developments
- Novartis/Yisheng Biotech Collaboration:
- Automated MolPure® systems for ASO/siRNA screening (processing 10,000 samples/day)
- QIAGEN/RNAscope® Integration:
- AI-powered digital pathology platforms for FFPE biomarker quantification
V. Frontier Innovations (2030 Horizon)
A. In Vivo Nanosensors
- Implantable Microdevices:
- Wireless RNA probes monitoring cytokine storms in sepsis patients
- Mitochondrial RNA Editors:
- CRISPR-free base editing probes correcting Parkinson’s-associated mutations
B. Synthetic Biology Interfaces
- RNA-Driven Biocomputing:
- Ribozyme-based logic gates processing cellular inputs
- Evolutionary Probes:
- Phage display-derived ligands targeting antibiotic resistance RNAs
(Fig. 3: Implantable RNA-sensing microchip)
Description: 5mm subcutaneous device with frankenbody probes (purple) transmitting real-time inflammation data to smartphone.
Conclusion: The RNA-Centric Medical Revolution
RNA probes will transform biomedical science through four converging revolutions:
- Chemical Intelligence – Machine-designed probes targeting “undruggable” RNA structures
- Dynamic Visualization – Ångstrom-resolution imaging of RNA translation in living tissue
- Automated Precision – AI-integrated platforms enabling clinic-to-bench reverse translation
- Continuous Monitoring – Implantable nanosensors providing real-time disease tracking
“We stand at the inflection point where RNA probes evolve from detection tools to autonomous cellular surgeons – capable of diagnosing dysregulation, executing targeted interventions, and verifying therapeutic efficacy within single living cells.”
— Nature Biotechnology (2025)By 2030, RNA probe technologies will penetrate >40% of IVD markets and enable first-in-class treatments for 30+ genetic disorders previously deemed incurable.
Data sourced from publicly available references. For collaboration or domain acquisition inquiries, contact: chuanchuan810@gmail.com.
- Cryo-EM Correlative Mapping:
