 1. Core Definition and Fundamental Principles
1. Core Definition and Fundamental Principles
Synthetic vaccines are a class of vaccines engineered de novo using synthetic biology, computational design, and chemical synthesis. Unlike traditional vaccines (live-attenuated or inactivated pathogens), they comprise synthetic components such as:
- Peptides: Short amino acid chains mimicking pathogen antigens .
- Nucleic acids: DNA or mRNA encoding target antigens .
- Polysaccharides/conjugates: Chemically synthesized carbohydrate antigens linked to carrier proteins .
Key distinction: They avoid biological pathogen material, eliminating risks of accidental infection and simplifying manufacturing .
Suggested Figure 1: Molecular Components of Synthetic Vaccines
- Left: Synthetic peptide (blue) bound to MHC complex (gray).
- Right: mRNA vaccine lipid nanoparticle (gold) delivering antigen code to cells.
2. Design Methodologies and Engineering Platforms
A. Rational Computational Design
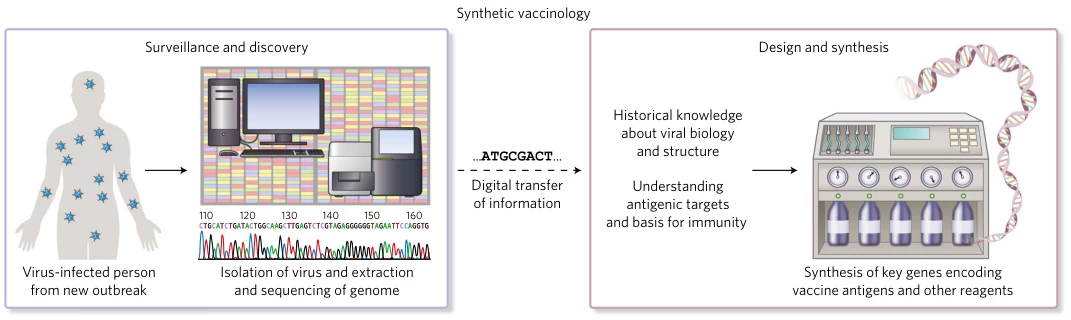
- SynRAD Engine: Proprietary platform (SynVaccine Inc.) designs viral genomes from scratch using big data and synthetic biology. It preserves critical features (e.g., immunogenicity) while introducing silent mutations to attenuate virulence .
- Structural Bioinformatics: Algorithms predict antigenic epitopes from pathogen genomes (e.g., SARS-CoV-2 spike protein) for precise peptide synthesis .
B. Modular Assembly Techniques
- SpyTag/SpyCatcher System: Covalent protein assembly enables rapid construction of multi-antigen vaccines .
- “Click Chemistry”: Orthogonal reactions (e.g., copper-free azide-alkyne cycloaddition) create monodisperse glycoconjugates for reproducible immune responses .
Suggested Figure 2: SynRAD Workflow
Pathogen sequence → In silico genome redesign → Chemical synthesis → Attenuated synthetic virus.
3. Advantages Over Traditional Vaccines
| Feature | Synthetic Vaccines | Traditional Vaccines |
|---|---|---|
| Safety | No pathogen handling; minimal contaminants | Risk of incomplete inactivation |
| Speed | 6–8 weeks from genomic data to candidate | Years for culture-based methods |
| Precision | Epitope-specific targeting | Whole-pathogen approach |
| Thermostability | DNA/mRNA candidates tolerate higher temps | Cold chain required |
Clinical Impact:
- COVID-19 mRNA vaccines (Pfizer/Moderna) achieved 95% efficacy in <1 year .
- SynVaccine’s candidates for Dengue/Ebola target conserved viral regions to prevent escape mutations .
4. Applications Across Diseases
A. Infectious Diseases
- COVID-19: mRNA vaccines encode spike protein variants .
- Malaria: Synthetic peptide vaccines (e.g., PfCP2.9) target Plasmodium antigens .
- HIV: DNA vaccines express conserved Env/Gag epitopes .
B. Cancer Immunotherapy
- Neoantigen Vaccines: Custom peptides from tumor mutations prime T-cell responses .
- Example: Fudan University’s SARS-CoV-2 RBD-Fc vaccine induced neutralizing antibodies .
C. Platform Versatility
- Virus-Like Particles (VLPs): Self-assembling synthetic capsids present antigens without genetic material .
- Nucleic Acid Platforms: Rapidly adaptable to emerging variants (e.g., Omicron boosters) .
Suggested Figure 3: Synthetic Vaccine Applications
- Top: mRNA-LNP entering cell (purple) for antigen expression.
- Bottom: Peptide-conjugate (orange) activating dendritic cells (green).
5. Challenges and Innovations
A. Current Limitations
- Immunogenicity: Synthetic peptides often require adjuvants (e.g., TLR2/6 agonists) .
- Delivery: LNPs enhance mRNA stability but face liver tropism limitations .
B. Cutting-Edge Solutions
- AI-Driven Optimization: CRISPR-TAPE selects conserved residues to minimize escape mutations .
- Codon-Deoptimization: Synonymous mutations attenuate viruses while preserving immunogenicity .
6. Future Directions
- Universal Pathogen Shields: Multi-epitope vaccines targeting conserved regions of virus families (e.g., Coronaviridae) .
- Needle-Free Delivery: Microneedle patches with lyophilized mRNA vaccines for low-resource settings .
- Real-Time Pandemic Response: On-site gene synthesis facilities enabling “just-in-time” vaccine production .
Conclusion
Synthetic vaccines represent a paradigm shift in vaccinology, merging computational design, synthetic biology, and nanotechnology. Their modularity enables rapid response to emerging pathogens, while precision engineering minimizes off-target effects. As platforms mature, they will democratize access to vaccines against intractable diseases—from Ebola to personalized cancer neoantigens—ushering in an era of “vaccines on demand.”
Data Source: Publicly available references.
Contact: chuanchuan810@gmail.com
