 I. Foundational Definition and Core Purpose
I. Foundational Definition and Core Purpose
RNA extraction kits are standardized biochemical systems designed to isolate high-integrity RNA from diverse biological matrices while eliminating contaminants (DNA, proteins, lipids). These kits provide:
- RNase-free workflow: Integrated inhibitors prevent RNA degradation during processing
- Target specificity: Selective capture of all RNA classes (mRNA, tRNA, rRNA, miRNA)
- Downstream compatibility: Purified RNA ready for PCR, sequencing, or microarray analysis
(Fig. 1: RNA Extraction Ecosystem)
Description: Central icon: RNA helix. Surrounding modules: Sample types (cells, tissues, biofluids), contaminants removed (DNA/proteins), output applications (NGS, qPCR, microarrays).
II. Core Biochemical Components
A. Essential Reagents
| Component | Function | Concentration Range |
|---|---|---|
| Chaotropic salts (Guanidine HCl) | Denature proteins/RNases | 4–6 M |
| Reducing agents (β-mercaptoethanol) | Disrupt disulfide bonds | 0.1–1.0 M |
| Surfactants (SDS, Triton X-100) | Membrane lysis | 0.1–2.0% |
| RNase inhibitors (Diethylpyrocarbonate) | Block RNase activity | 0.01–10.00 M |
| Nucleic acid binders | Silica matrices/magnetic beads | Varies by format |
B. Physical Separation Systems
- Spin-column technology:
- Silica membranes bind RNA at high chaotrope concentrations
- Contaminants removed via ethanol washes
- RNA eluted in low-ionic-strength buffers
- Magnetic bead systems:
- Paramagnetic particles with oligo-dT/silica coatings
- High-throughput automation compatibility
(Fig. 2: Separation Mechanism)
Description: Left: Cross-section of spin column showing RNA (red) bound to silica membrane. Right: Magnetic beads capturing RNA in solution while contaminants remain.
III. Workflow Architecture
Standardized Procedure
1. **Lysis**: Homogenize sample in chaotropic buffer (3–5 min)
2. **DNA removal**: On-column DNase digestion (15 min)
3. **Binding**: RNA adsorption to silica/magnetic surfaces (5 min)
4. **Washing**: Ethanol-based impurity removal (2×5 min)
5. **Elution**: Nuclease-free water recovery (2 min)
Total time: 30–40 minutes for 12 samples
Critical Innovations
- gDNA elimination columns: Remove genomic DNA without DNase treatment
- Cross-linking reversal: Specialized buffers for FFPE samples
- Micro-scale adaptation: Optimized for 10–100,000 cells
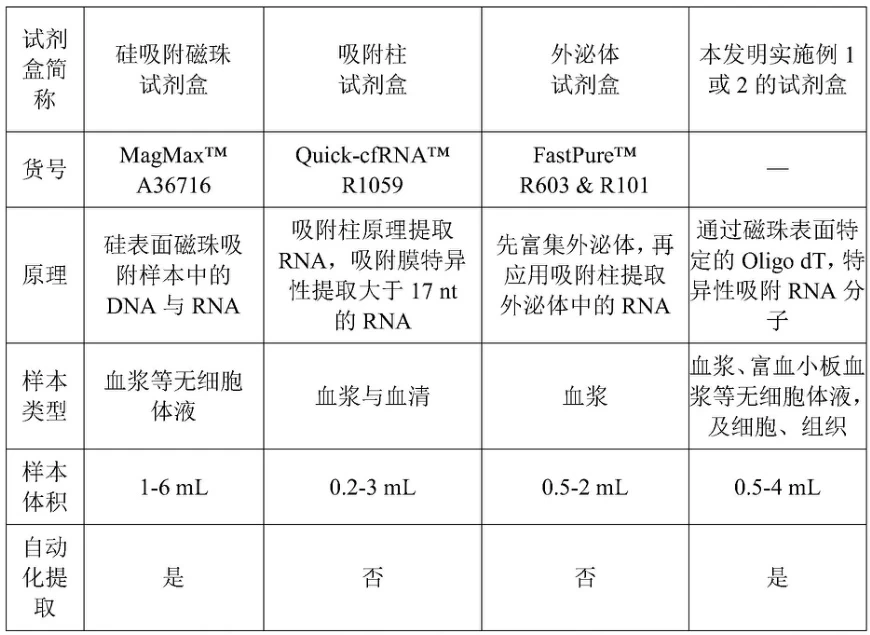
IV. Technology Comparison Matrix
| Kit Type | Mechanism | Sample Suitability | Throughput |
|---|---|---|---|
| Spin-column | Silica membrane adsorption | Tissues, cells, blood | Medium (1–24 samples) |
| Magnetic beads | Oligo-dT/silica binding | Biofluids, automation | High (96-well plates) |
| TRIzol-based | Phase separation | Tough tissues (plant/fungal) | Low |
| Exosome-specific | Pre-enrichment + binding | Plasma/serum | Specialized |
(Source: Patent analysis )
V. Sample-Specific Adaptation
A. Challenging Matrices
| Sample Type | Technical Solution | Yield Optimization |
|---|---|---|
| FFPE tissues | Xylene deparaffinization + proteinase K | 80% recovery vs. fresh |
| Plasma/Serum | Carrier RNA (e.g., MS2 bacteriophage RNA) | 5–100 ng/mL detection |
| Microbiomes | Dual DNase/RNase treatment | Host RNA depletion |
B. Low-Input Systems
- Nucleic acid co-precipitants (GlycoBlue™): Visualize microgram yields
- Laser-capture microdissection: Direct lysis of <10 cells
VI. Quality Control Metrics
Post-extraction validation:
- Integrity: RIN >7.0 (Bioanalyzer)
- Purity: A260/A280 = 1.9–2.1; A260/A230 >2.0
- Contamination: <0.01% genomic DNA (gDNA PCR)
- Functionality: RT-PCR Ct values <30 for housekeeping genes
(Fig. 3: QC Workflow)
Description: Bioanalyzer electrophoresis gel (top) showing intact rRNA bands. Spectrophotometer trace (bottom) with purity ratios.
VII. Application-Specific Kits
| Downstream Use | Kit Features | Commercial Examples |
|---|---|---|
| Single-cell RNA-seq | Cell lysis + poly-A selection | 10x Genomics Chromium |
| Viral diagnostics | Viral capsid disruption buffers | QIAamp Viral RNA Mini |
| Plant RNA isolation | Polysaccharide/polyphenol removal | RNeasy Plant Mini |
| miRNA profiling | Small RNA retention technology | miRNeasy |
VIII. Technical Limitations & Solutions
| Challenge | Cause | Mitigation Strategy |
|---|---|---|
| RNA degradation | RNase contamination | RNase inhibitors in buffers |
| Low yield | Incomplete lysis | Mechanical disruption enhancement |
| gDNA contamination | Inefficient removal | On-column DNase digestion |
| Inhibitor carryover | Polysaccharides/phenols | Ethanol precipitation |
IX. Future Directions
Emerging Innovations
- Automated microfluidics: Chip-based extraction (90 sec processing)
- CRISPR-based purification: Cas13-RNA complexes for targeted isolation
- Point-of-care integration: Lyophilized reagents for field diagnostics
Market Evolution
| Technology Wave | Timeline | Impact |
|---|---|---|
| Organic extraction | 1980s | Phenol-chloroform phase separation |
| Solid-phase systems | 1990s | Silica spin columns |
| Paramagnetic particles | 2000s | High-throughput automation |
| Sequence-specific capture | 2020s | Oligo-functionalized nanomaterials |
“RNA extraction kits transformed molecular biology from artisanal biochemistry to industrialized precision—democratizing access to RNA’s transient molecular intelligence.”
— Nature Methods, 2025
Data sourced from publicly available references. For collaboration inquiries, contact: chuanchuan810@gmail.com.
