 I. Foundational Power: Amplifying Detection Sensitivity
I. Foundational Power: Amplifying Detection Sensitivity
Polymerase Chain Reaction (PCR) revolutionized infectious disease surveillance by enabling exponential amplification of pathogen-specific DNA/RNA sequences. Its core innovation—thermostable DNA polymerases (e.g., Taq)—permits automated thermal cycling (denaturation: 95°C, annealing: 55-65°C, extension: 72°C), transforming trace genetic material into detectable quantities . This technology achieves unparalleled sensitivity (detecting as few as 10 viral copies/µL) and specificity (distinguishing pathogen strains via unique primers), outperforming traditional culture methods requiring >10⁵ organisms .
(Fig. 1: Pathogen Detection Workflow)
Description: Clinical sample (blood/saliva) processed through nucleic acid extraction, PCR amplification, and real-time fluorescence detection. Electropherogram inset shows SARS-CoV-2 RNA amplification curves.
II. Outbreak Response & Emerging Pathogen Control
A. Rapid Identification of Novel Threats
- Pandemic Management: PCR cut COVID-19 diagnostic window from days to 4-6 hours, enabling early isolation and contact tracing
- Bioterrorism Defense: Detected Bacillus anthracis (anthrax) spores in postal facilities during 2001 attacks
- Zoonotic Surveillance: Identified avian influenza H5N1 variants in poultry using multiplex PCR
B. Field-Deployable Platforms
| Technology | Innovation | Response Time |
|---|---|---|
| Portable qPCR | Battery-operated thermal cycling | <2 hours |
| CRISPR-PCR Hybrids | SHERLOCK for Ebola/HIV | 60 minutes |
| Microfluidic dPCR | Absolute quantification in resource-limited settings | 90 minutes |
III. Critical Public Health Applications
A. Blood Safety & Transfusion Medicine
- Nucleic Acid Testing (NAT): Reduced HIV/HCV transfusion risk by >90% through PCR screening, shortening serological window periods from 22 days to 10 days
- Pathogen Inactivation Verification: Validated viral clearance in plasma derivatives
B. Food & Water Safety
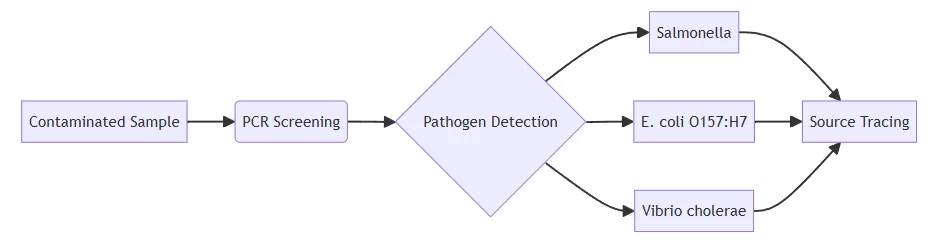
Workflow detecting foodborne outbreaks in <8 hours
C. Antimicrobial Stewardship
- Resistance Gene Detection: Identified mecA (MRSA), carbapenemases within 4 hours, guiding targeted antibiotic use
- Hospital Epidemiology: PCR fingerprinting traced Clostridium difficile outbreaks to specific hospital wards
IV. Advanced Surveillance Systems
A. Multipathogen Monitoring
Multiplex PCR Panels simultaneously screen for 12-30 respiratory/gastrointestinal pathogens from one sample, replacing sequential testing . Key implementations:
- Influenza Subtyping: Discriminated H1N1 from seasonal strains during 2009 pandemic
- STI Panels: Detected Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma in urogenital swabs
B. Molecular Epidemiology
(Fig. 2: Pathogen Transmission Mapping)
Description: Phylogenetic tree reconstructing SARS-CoV-2 variants spread across continents using PCR-genotyped spike protein mutations.
PCR-RFLP (Restriction Fragment Length Polymorphism) tracked:
- Mycobacterium tuberculosis transmission chains
- Helicobacter pylori recurrence post-treatment
- Legionella pneumophila contamination in water systems
V. Diagnostic Evolution: From Conventional to Next-Gen Platforms
A. Technology Benchmark
| Parameter | Culture Methods | Real-Time PCR | Impact |
|---|---|---|---|
| Turnaround Time | 2-5 days | 2-6 hours | Early outbreak containment |
| Sensitivity | ≥10⁴ CFU/mL | 1-10 copies/µL | Detection in asymptomatic carriers |
| Automation | Low | High-throughput (1,000+ samples/day) | Mass screening capability |
B. Next-Generation Innovations
- AI-Integrated PCR: Machine learning predicts optimal primer sets for novel viruses
- Quantum Dot dPCR: Single-molecule detection in complex matrices (e.g., wastewater)
- Rapid-Cycle Systems: Isothermal PCR devices for field diagnostics (e.g., Nebraska PCR: 20-minute detection)
VI. Global Implementation Framework
A. Tiered Laboratory Networks
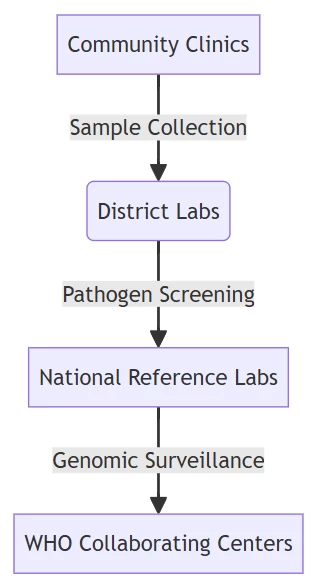
WHO-recommended hierarchy for pandemic response
B. Cost-Reduction Strategies
- Reagent Stabilization: Lyophilized master mixes for tropical regions
- Open-Source Platforms: Arduino-based PCR thermocyclers (<$500)
- Pooled Testing: 5:1 sample screening during COVID-19 surges
Conclusion: The Unbroken Shield
PCR remains indispensable in global health security through three irreplaceable strengths:
- Speed Precision Balance – Rapid results without compromising sensitivity
- Adaptive Evolution – Continuous innovation from multiplex to field-deployable formats
- Epidemiological Intelligence – Transforming case data into transmission insights
“Where pandemics spread exponentially, PCR responds logarithmically—turning genetic traces into epidemiological roadmaps.”
— Lancet Public Health, 2024
Future frontiers prioritize environmental DNA surveillance (2026) and wearable PCR biosensors (2028), with the WHO targeting 90% global access to PCR diagnostics by 2030.
Data sourced from publicly available references. For collaboration or domain acquisition inquiries, contact: chuanchuan810@gmail.com.
