 I. Foundational Technology: Amplifying Diagnostic Sensitivity
I. Foundational Technology: Amplifying Diagnostic Sensitivity
Polymerase Chain Reaction (PCR) revolutionizes disease screening by enabling exponential amplification of target DNA/RNA sequences from minimal biological samples. Its core methodology—thermal cycling (denaturation: 95°C, annealing: 55-65°C, extension: 72°C)—combined with thermostable polymerases (Taq) achieves sensitivities of 1-10 copies/µL, outperforming traditional culture and serological methods .
(Fig. 1: Universal Screening Workflow)
Description: Integrated pipeline showing sample collection (blood/tissue/swab), nucleic acid extraction, PCR amplification, and detection via electrophoresis or fluorescence. Multiplex PCR panel inset identifies 12 pathogens simultaneously.
II. Infectious Disease Screening: Rapid Pathogen Identification
A. Multiplex Pathogen Detection
Simultaneous screening of respiratory, gastrointestinal, and sexually transmitted infections (STIs) using multiplex PCR panels:
- Respiratory Pathogens: Influenza A/B, SARS-CoV-2, RSV in <2 hours
- STI Panels: Chlamydia trachomatis, Neisseria gonorrhoeae, HPV with 99% specificity
- Blood Safety: HIV/HBV/HCV nucleic acid testing (NAT) reduces transfusion transmission risk by >90%
B. Antibiotic Resistance Profiling
| Target | PCR Method | Clinical Impact |
|---|---|---|
| mecA (MRSA) | Real-time PCR | Guides antibiotic stewardship |
| Carbapenemases | Multiplex PCR | Identifies drug-resistant Gram-negatives |
| rpoB (TB) | Nested PCR | Detects rifampicin resistance |
III. Cancer Risk Stratification: Molecular Early Warning
A. Hereditary Cancer Syndromes
- BRCA1/2 Screening: Allele-specific PCR detects pathogenic variants (e.g., 185delAG) in high-risk populations
- PIK3CA Hotspots: ARMS-PCR identifies E542K/E545K/H1047R mutations for breast cancer therapy selection
- Lynch Syndrome: Microsatellite instability (MSI) testing via PCR fragment analysis
B. Liquid Biopsy Applications
Digital PCR (dPCR) enables non-invasive cancer screening:
- Circulating Tumor DNA (ctDNA): Detects EGFR T790M at 0.1% allele frequency
- Minimal Residual Disease: Identifies 1 malignant cell/10⁶ in leukemia
(Fig. 2: dPCR Workflow)
Description: Plasma separation (left), microfluidic partitioning into 20,000 droplets (center), target amplification (right) enabling absolute ctDNA quantification.
IV. Genetic Disorder Screening: Preventing Hereditary Diseases
A. Prenatal/Newborn Testing
- SMA Screening: dPCR quantifies SMN1/SMN2 copy numbers with CE-IVD certification
- Cystic Fibrosis: Multiplex PCR detects ΔF508, G542X mutations in 99% carriers
- Thalassemia: Gap-PCR identifies α-globin gene deletions
B. Carrier Screening Programs
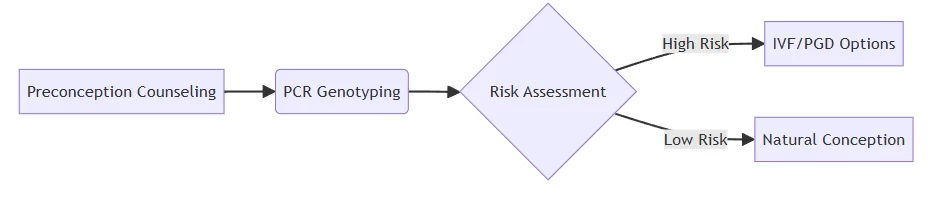
Preventive pathway for recessive disorders
V. Technology Selection Matrix
A. Method-Specific Applications
| Screening Scenario | Optimal PCR Format | Turnaround Time |
|---|---|---|
| Pandemic Surveillance | Real-time multiplex PCR | 2-4 hours |
| Oncology ctDNA | Digital PCR | 6 hours |
| Neonatal SMA | dPCR with CE-IVD kits | 3 hours |
| STI Point-of-Care | Isothermal LAMP | 30 minutes |
B. Performance Benchmark
| Parameter | Conventional PCR | dPCR | Clinical Advantage |
|---|---|---|---|
| Sensitivity | 1,000 copies | 1 copy | Early-stage detection |
| Quantitative Accuracy | Relative | Absolute | Therapy monitoring |
| Multiplex Capacity | Low (3-5 targets) | High (10-30 targets) | Comprehensive screening |
VI. Quality Assurance Framework
A. Critical Control Points
- Pre-analytical: Standardized sample collection (e.g., EDTA blood for viral load)
- Analytical: Internal amplification controls (IACs) for inhibitor detection
- Post-analytical: ΔC<sub>q</sub> analysis to confirm amplification efficiency
B. Contamination Mitigation
- Physical Separation: Pre-PCR, amplification, and post-PCR zones
- Enzymatic Controls: Uracil-DNA glycosylase (UNG) prevents amplicon carryover
- Automated Systems: Integrated extraction-amplification platforms
Conclusion: Transforming Prevention Paradigms
PCR screening redefines disease prevention through:
- Anticipatory Diagnostics – Detecting molecular abnormalities before symptom onset
- Precision Stratification – Quantifying risk via genetic variants and pathogen loads
- Accessible Technology – From CE-IVD kits to point-of-care LAMP devices
“Where traditional screening sees shadows, PCR illuminates molecular footprints—turning prevention from aspiration into actionable science.”
— Nature Reviews Diagnostics, 2025
Future innovations prioritize CRISPR-integrated PCR for single-mutation detection (2026) and AI-interpretive platforms (2028), with the global PCR screening market projected to reach $28.4B by 2030 .
Data sourced from publicly available references. For collaboration or domain acquisition inquiries, contact: chuanchuan810@gmail.com.
