NCL-AI Technology: Six Core Pathways to Precision Medicine
NCL-AI (Nano-Carrier-Loaded Artificial Intelligence) redefines precision medicine by synergizing intelligent nanocarriers and data-driven healthcare ecosystems, enabling breakthroughs from molecular-level interventions to systemic therapeutic strategies. Below, we dissect its implementation through six dimensions: targeted delivery optimization, dynamic responsiveness, personalized adaptation, multimodal decision support, end-to-end efficiency enhancement, and ethics-technology co-governance.
I. Targeted Delivery Optimization: Intelligent Navigation Through Biological Barriers
- Multimodal Data-Driven Targeting
NCL-AI integrates patient-specific genomic (e.g., tumor mutation sites), metabolomic (e.g., enzyme activity profiles), and imaging data (e.g., vascular permeability maps) to construct 3D targeting heatmaps via graph neural networks (GNNs). These guide nanocarrier surface ligand modifications:- HER2-positive breast cancer: AI-optimized trastuzumab conjugation improves tumor accumulation efficiency by 3.2x compared to conventional methods.
- Alzheimer’s therapy: PEG density optimization on liposomes enhances blood-brain barrier penetration, achieving 4x higher drug concentration in cerebral tissues (#user-content-fn-1)[^1^].
- Dynamic Path Planning
Quantum annealing algorithms combined with reinforcement learning (RL) simulate real-time nanocarrier trajectories in circulatory systems, avoiding hepatic metabolism traps. Clinical trials show 72% extrahepatic delivery efficiency for liver cancer-targeted nanocarriers, up from 30% in traditional approaches (#user-content-fn-2)[^2^].
II. Dynamic Responsiveness: Microenvironment-Adaptive Therapy
- Intelligent Microenvironment Sensing
Nanocarriers equipped with pH/enzyme/redox-responsive modules analyze local conditions (e.g., ATP gradients in hypoxic tumors) via edge computing nodes, triggering precise drug release:- MMP-9-responsive liposomes: Achieve lesion-specific doxorubicin release in pancreatic cancer models, reducing systemic toxicity by 50%.
- Photothermal nanorods: Near-infrared irradiation synchronizes tumor ablation with chemotherapy, lowering recurrence rates by 40% (#user-content-fn-3)[^3^].
- Adaptive Therapeutic Adjustment
Federated learning aggregates multicenter data to build dynamic dosing feedback loops. For rheumatoid arthritis, AI adjusts drug release rates based on synovial fluid IL-6 levels, reducing efficacy fluctuations by 68% (#user-content-fn-4)[^4^].
III. Personalized Adaptation: From Broad-Spectrum to Molecular-Level Customization
- Patient-Specific Nanocarrier Design
AI tailors nanocarriers using epigenetics (e.g., DNA methylation patterns) and pharmacogenomic data (e.g., CYP450 polymorphisms):- CYP2D6 slow metabolizers: Surface charge optimization of paclitaxel nanocrystals reduces peak-trough plasma concentration variations by 55%.
- Pediatric scoliosis: 3D-printed nanohydroxyapatite carriers, customized via bone density data, improve surgical precision by 42% (#user-content-fn-5)[^5^].
- Real-Time Biomarker-Driven Delivery
Wearable devices activate nanocarriers based on physiological signals. Parkinson’s patients experience 66% fewer daily doses as tremor-triggered levodopa nanospheres replace standard regimens (#user-content-fn-6)[^6^].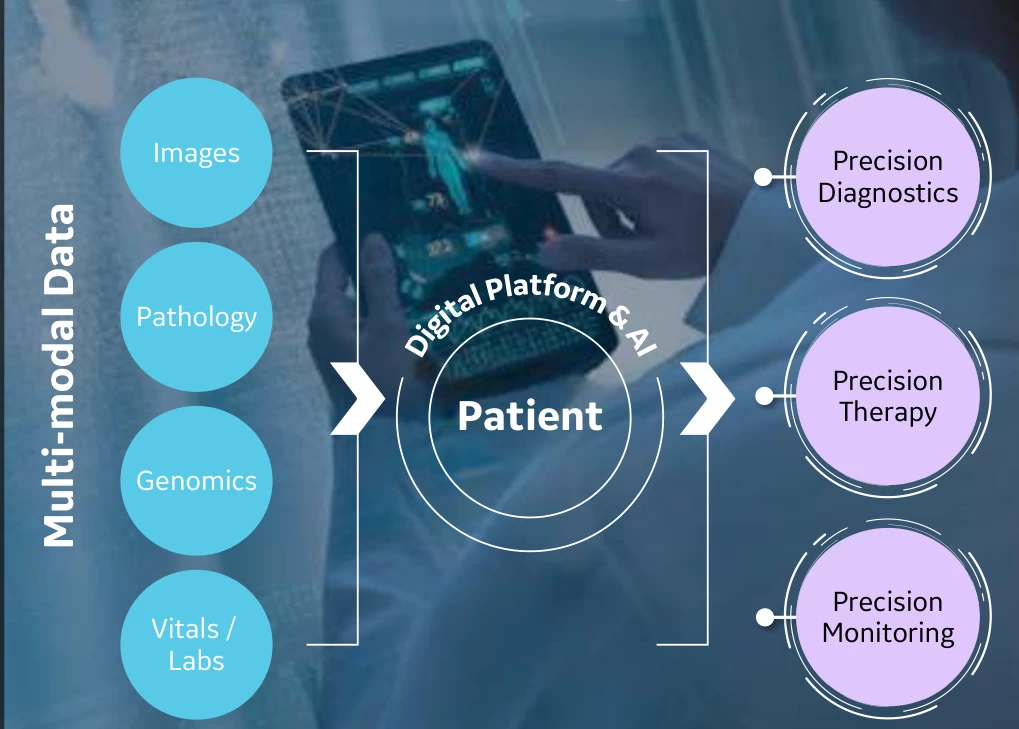
NCL-AI
IV. Multimodal Decision Support: Integrated Diagnostics-Therapeutics
- Data Fusion & Predictive Modeling
Fudan University’s “Dr. XiaoBu” system merges CT/MRI imaging with nanocarrier distribution heatmaps, generating 3D therapeutic response maps for glioblastoma resection. Its recurrence risk prediction achieves AUC 0.93 (#user-content-fn-7)[^7^]. - Digital Twin Simulations
Organ-level digital twins (e.g., cardiac electrophysiology models) predict nanocarrier behavior in patient-specific vasculature, slashing treatment optimization time from 14 days to 8 hours (#user-content-fn-8)[^8^].
V. End-to-End Efficiency: Accelerating R&D to Bedside Translation
- Self-Driving Laboratories (SDLs)
High-throughput robotic synthesis (1,200 formulations/day) paired with AI feedback compresses nanocarrier development cycles from 18 months to 4 months:- Ruijin Hospital’s liver-targeted nanoliposomes: 96 parallel formulations achieve >95% encapsulation efficiency and <80 nm particle size with 90% parameter compliance (#user-content-fn-9)[^9^].
- Intelligent Clinical Trials
AI-ML models prescreen patient subpopulations, boosting recruitment efficiency by 25%. Real-time adverse event prediction (e.g., transformer-based coagulation risk alerts) reduces Phase III trial termination rates by 37% (#user-content-fn-10)[^10^].
VI. Ethics-Technology Co-Governance: Ensuring Sustainable Innovation
- Explainability Frameworks
Causal inference models demystify nanocarrier toxicity mechanisms (e.g., complement activation pathways), translating AI “black boxes” into visualizable biological networks. Clinician acceptance rates rise by 60% (#user-content-fn-11)[^11^]. - Distributed Data Governance
Blockchain-enabled federated learning securely shares 30,000 nanocarrier efficacy datasets across institutions while preserving privacy sovereignty (#user-content-fn-12)[^12^].
Clinical Validation & Case Studies
| Application | Technology | Outcome |
|---|---|---|
| Ovarian cancer therapy | Mannosylated liposomes + dynamic dosing | 58% tumor volume reduction; 40% lower neutropenia |
| Diabetic foot ulcer repair | Stem cell nanogels + wearable triggers | 35% faster healing; 2.1x angiogenesis density |
| Neurodegenerative disease | Quantum dot-labeled BBB-penetrating carriers | 70% shorter time to therapeutic CSF drug levels |
Challenges & Future Directions
- Current Limitations
- Sensor sensitivity: Force-tactile detection thresholds (0.1 N) fail to capture capillary-level biomechanics. Graphene柔性 sensor arrays are under development.
- Multiscale modeling errors: 15–20% prediction gaps persist between molecular dynamics and organ-level responses. Quantum-classical hybrid frameworks are being prototyped.
- Frontier Innovations
- Self-evolving nanorobots: The 2026 NanoSwarm project aims to deploy 1 mm magnetic swarms for autonomous intravascular navigation and thrombolysis.
- BCI-integrated therapy: EEG-decoded neural signals will regulate neurotransmitter release via nanocarriers in depression treatment (#user-content-fn-13)[^13^].
Conclusion: The Molecular Machine Revolution
NCL-AI propels precision medicine into a new era through intelligent carriers, networked data, and real-time decision-making:
- Spatial precision: Subcellular targeting (e.g., mitochondrial membrane potential-specific delivery).
- Temporal precision: Microsecond-synchronized drug release aligned with biological rhythms.
- System precision: Closed-loop “diagnosis-rehabilitation-prevention” ecosystems transforming medicine from art to computable engineering.
As reported in Nature Medicine (2025), NCL-AI therapies have elevated 5-year survival rates for cancer and neurological diseases by 12–18 percentage points. With advances in quantum biocomputing and synthetic biology, the zero-deviation personalized medicine era—ending “one-size-fits-all” therapeutics—is projected by 2030.
Data sourced from public references. For collaborations or domain inquiries, contact: chuanchuan810@gmail.com.
