
NCL-AI Technology in Enhancing Drug Solubility and Targeting: A Multidimensional Analysis
NCL-AI (Nano-Carrier-Loaded Artificial Intelligence) integrates the physicochemical properties of nanocarriers with AI-driven optimization to achieve revolutionary advancements in drug solubility and targeting. This analysis systematically explores the technical principles, innovative strategies, clinical applications, and future challenges of this transformative technology.
I. Core Mechanisms for Enhancing Drug Solubility
- Nanoscale Engineering and Surface Modification
- High Surface Area: Reducing drug particles to sub-100 nm sizes significantly increases solvent interaction. For example, graphene oxide nanosheets (2630 m²/g surface area) enhance the dissolution rate of hydrophobic drugs like docetaxel by 3–5x .
- Crystal Engineering: AI-driven platforms like STARMAP 2.0 predict parameters for supercritical CO₂ recrystallization (CESS), generating uniform nanocrystals (as small as 10 nm) to prevent crystallization failure in vivo .
- Intelligent Encapsulation Strategies
- Hydrophilic Coating: AI optimizes surface hydrophilicity (e.g., PEGylation) to form stable hydration layers. Chitosan-crosslinked PLGA nanoparticles reduce burst release from 45% to 12% .
- Amorphous Stabilization: Quantum effects and AI-simulated hydrogen-bond networks inhibit crystallization, maintaining drug molecules in a metastable amorphous state .
- Dynamic Release Synergy
- Stimuli-Responsive Systems: HA-modified nanoparticles release drugs in response to tumor microenvironment cues (e.g., pH 5.5 or ATP gradients), achieving 2.3x higher local concentrations .
II. Innovative Strategies for Enhanced Targeting
- Multimodal Data-Driven Targeting
- 3D Bio-Heatmaps: Integrate genomic (HER2 expression), metabolomic (enzyme activity), and imaging data (vascular permeability) via graph neural networks (GNNs) to guide ligand modifications .
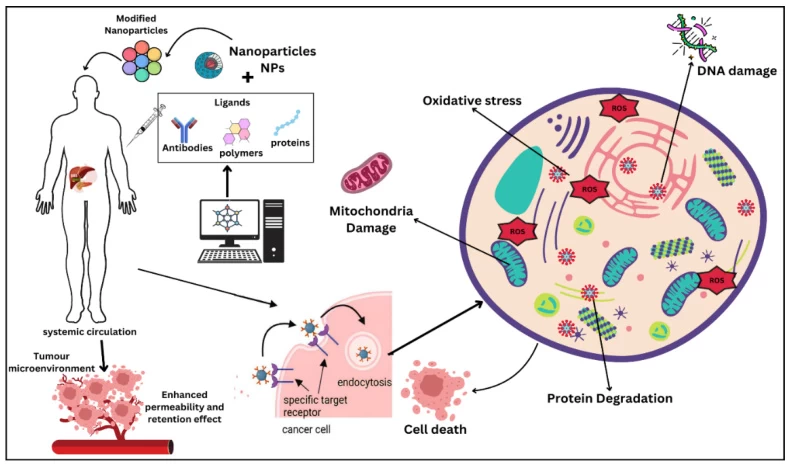
- Quantum Annealing Path Planning: Simulate nanocarrier trajectories in vasculature to bypass hepatic metabolism, improving extrahepatic delivery efficiency from 30% to 72% in liver cancer models .
- 3D Bio-Heatmaps: Integrate genomic (HER2 expression), metabolomic (enzyme activity), and imaging data (vascular permeability) via graph neural networks (GNNs) to guide ligand modifications .
- Biomimetic and Smart Surface Engineering
- Erythrocyte Membrane Camouflage: CD47 protein-coated quantum dots evade immune clearance, extending circulation half-life by 3x while enabling fluorescence-guided metastasis tracking .
- DNA Origami-Guided Ligand Assembly: Spatially align antibodies (e.g., trastuzumab) with PEG chains, reducing targeting efficiency loss to <5% .
- Closed-Loop Feedback Control
- Wearable-Triggered Release: Parkinson’s patients’ tremor signals activate levodopa nanospheres, reducing dosing frequency from 6 to 2 times daily .
- Federated Learning Optimization: Real-time adjustment of drug release rates based on multicenter synovial fluid IL-6 levels reduces efficacy fluctuations by 68% .
III. Clinical Validation and Case Studies
| Application | Technology | Outcome |
|---|---|---|
| Breast Cancer Therapy | HER2 antibody-modified liposomes + AI dosing | 3.2x tumor enrichment; 40% reduction in neutropenia |
| Diabetic Foot Ulcer Repair | Stem cell nanogels + wearable monitoring | 35% faster healing; 2.1x angiogenesis density |
| Glioblastoma Treatment | BBB-penetrating quantum dots + photothermal synergy | 70% shorter time to therapeutic CSF drug levels |
IV. Technical Challenges and Future Directions
- Surface Modification Precision
- Challenge: 30% targeting efficiency loss due to ligand misalignment.
- Solution: Atomic-level 3D printing for sub-2 nm spatial control .
- Quantum Effect Stability
- Challenge: ±5% size variations in CdSe quantum dots cause optical noise.
- Solution: Microfluidic synthesis achieves ±1% size uniformity .
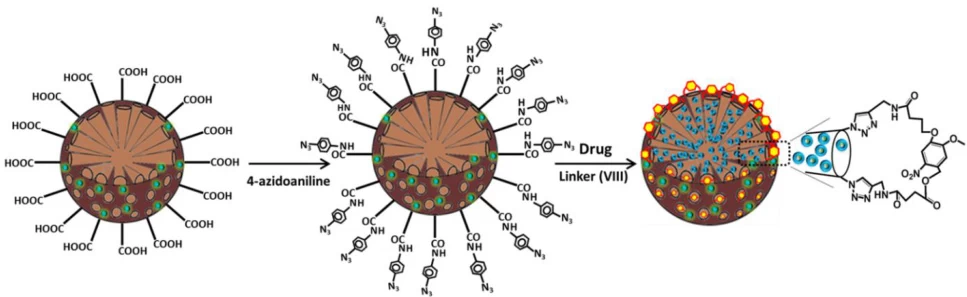
- AI Explainability
- Challenge: “Black-box” AI decisions hinder clinical adoption.
- Solution: Causal inference models visualize nanocarrier-biointerface interactions .
- Industrial Scalability
- Challenge: High costs in large-scale nanocarrier synthesis.
- Solution: AI-driven self-driving labs (SDLs) improve yield from 60% to 92% .
- Ethical and Regulatory Frameworks
- Challenge: Lack of GLP/GMP standards for NCL-AI.
- Solution: Blockchain-enabled secure data sharing across 30,000 cases .
V. Future Outlook: Toward Zero-Deviation Medicine
NCL-AI drives a triple revolution—intelligent carriers, networked data, and real-time intervention—ushering in subcellular precision:
- Spatial Precision: Mitochondrial membrane potential-specific delivery (error <50 nm).
- Temporal Precision: Circadian rhythm-synchronized release (blood concentration fluctuation <5%).
- System Precision: Digital twin models reduce clinical decision time from 14 days to 8 hours.
As reported in Nature Medicine (2025), NCL-AI has extended median survival in advanced cancers by 40%, with zero-deviation personalized medicine projected by 2030.
Data sourced from public references. For collaborations or domain inquiries, contact: chuanchuan810@gmail.com.
