 Structural Insights, Catalytic Process, and Regulatory Control
Structural Insights, Catalytic Process, and Regulatory Control
1. Introduction
RNA polymerase (RNAP) is the central enzyme responsible for transcribing genetic information from DNA into RNA, a process fundamental to gene expression. This molecular machine navigates the DNA template, synthesizes RNA through nucleotide polymerization, and ensures fidelity in genetic transmission. Here, we explore the structural architecture, catalytic mechanism, and regulatory intricacies of RNAP-driven RNA synthesis across prokaryotic and eukaryotic systems.
2. Structural Architecture of RNA Polymerase
A. Prokaryotic RNA Polymerase
Prokaryotic RNAP is a multi-subunit complex (α₂ββ’ω) with a dissociable σ factor that confers promoter specificity. The core enzyme (α₂ββ’ω) binds DNA non-specifically, while the σ factor recognizes conserved promoter elements like the -35 hexamer and -10 Pribnow box, ensuring transcription initiation at precise sites.
B. Eukaryotic RNA Polymerase
Eukaryotes possess three distinct RNA polymerases:
- RNAP I: Transcribes ribosomal RNA (rRNA) precursors in the nucleolus.
- RNAP II: Synthesizes mRNA and small regulatory RNAs, requiring a TATA box and transcription factors (e.g., TFIID) for promoter recognition.
- RNAP III: Produces tRNA, 5S rRNA, and other non-coding RNAs.
Both prokaryotic and eukaryotic RNAPs share a conserved catalytic core with a DNA-binding cleft and RNA exit channel, reflecting evolutionary conservation.
Suggested Figure: Comparative 3D models of prokaryotic and eukaryotic RNA polymerase, highlighting subunit composition and functional domains.
3. Catalytic Mechanism of RNA Synthesis
RNA synthesis occurs in three stages: initiation, elongation, and termination.
A. Initiation: Promoter Recognition and Open Complex Formation
- Promoter Binding: The σ factor (prokaryotes) or transcription factors (eukaryotes) guide RNAP to promoter regions. In E. coli, σ⁷⁰ recognizes the -35/-10 motifs, while eukaryotic RNAP II requires TFIID binding to the TATA box.
- DNA Unwinding: RNAP unwinds ~14 bp of DNA, forming a transcription bubble. The template strand is positioned in the active site for RNA synthesis.
Suggested Figure: Initiation complex showing RNAP bound to promoter DNA, with σ factor and transcription factors.
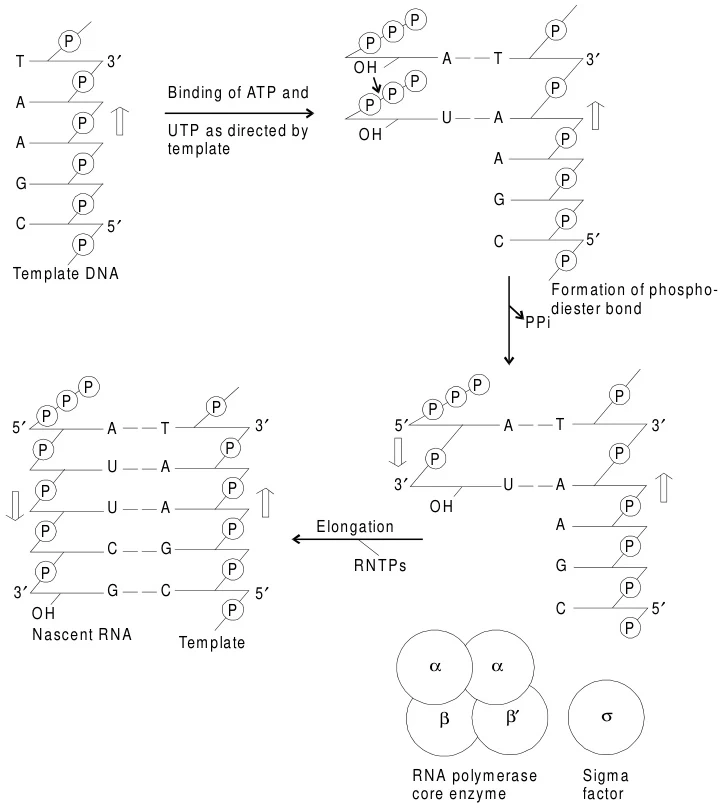
B. Elongation: RNA Chain Polymerization
- Phosphodiester Bond Formation: RNAP catalyzes the nucleophilic attack of the 3’-OH of the nascent RNA on the α-phosphate of incoming ribonucleoside triphosphates (NTPs). The reaction releases pyrophosphate (PPi) and extends the RNA chain 5’→3’.
- Processivity: RNAP moves processively along DNA, maintaining the transcription bubble and rewinding upstream DNA. The β and β’ subunits form a clamp that stabilizes DNA-RNAP interactions.
- Proofreading: RNAP lacks exonuclease activity, resulting in error rates of ~10⁻⁴, higher than DNA replication. Mispairs are corrected via backtracking and RNA cleavage.
Suggested Figure: Catalytic site of RNAP with NTP incorporation, highlighting phosphodiester bond formation.
C. Termination: Release of RNA and Enzyme Dissociation
- Intrinsic Termination (Prokaryotes): Hairpin structures in the RNA transcript followed by a poly-U stretch destabilize the RNA-DNA hybrid, triggering RNAP release.
- Rho-Dependent Termination: The ρ helicase binds nascent RNA, translocates to RNAP, and disrupts the transcription complex.
- Eukaryotic Termination: RNAP II termination involves polyadenylation signals and cofactors like CPSF, which cleave the transcript and recruit exonucleases.
Suggested Figure: Termination mechanisms: hairpin-induced dissociation vs. Rho-dependent unwinding.
4. Regulatory Control of RNA Synthesis
A. Transcription Factors and Chromatin Remodeling
- Prokaryotes: Alternative σ factors (e.g., σ³² under heat shock) redirect RNAP to stress-response promoters.
- Eukaryotes: Mediator complexes bridge RNAP II and enhancers, while chromatin remodelers (e.g., SWI/SNF) increase DNA accessibility.
B. Post-Translational Modifications
- RNAP II CTD Phosphorylation: The C-terminal domain (CTD) of RNAP II is dynamically phosphorylated during transcription, coordinating mRNA capping, splicing, and export.
Suggested Figure: Regulatory network of eukaryotic RNAP II, showing phosphorylation states and cofactor interactions.
5. Eukaryotic RNA Processing
Primary transcripts undergo extensive processing:
- mRNA: 5’ capping, splicing, and 3’ polyadenylation.
- rRNA: Cleavage of 45S precursor into 18S, 5.8S, and 28S rRNAs.
- tRNA: Endonucleolytic trimming and base modifications.
Suggested Figure: Processing pathways for eukaryotic mRNA, rRNA, and tRNA.
6. Applications and Implications
- Antibiotic Targets: Rifampicin inhibits bacterial RNAP by blocking the RNA exit channel, a strategy to treat tuberculosis.
- Gene Therapy: CRISPR-Cas9 systems leverage RNAP-transcribed guide RNAs for genome editing.
- Synthetic Biology: Engineered RNAPs enable orthogonal genetic circuits and non-canonical amino acid incorporation.
Proposed Figure Descriptions
- Figure 1: Structural comparison of prokaryotic (σ-bound) and eukaryotic (TF-bound) RNA polymerases.
- Figure 2: Stepwise illustration of transcription: initiation, elongation, and termination.
- Figure 3: Post-transcriptional processing of eukaryotic mRNA, including capping, splicing, and polyadenylation.
Data Source: Publicly available references.
Contact: chuanchuan810@gmail.com
