 A Multidimensional Exploration of Structural Regulation in the Genome
A Multidimensional Exploration of Structural Regulation in the Genome
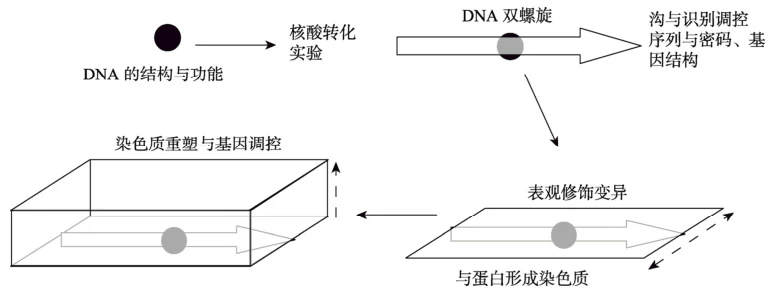
1. Introduction
DNA is far more than a static repository of genetic information; its dynamic architecture serves as a master regulator of gene expression. Beyond the iconic double helix, DNA adopts diverse conformations—from local secondary structures to chromatin-level 3D folding—that govern transcriptional accessibility, precision, and cellular identity. This article synthesizes cutting-edge research to elucidate how DNA’s hierarchical structure orchestrates gene expression across spatial and temporal scales, integrating insights from epigenetics, biophysics, and genome biology.
2. DNA Conformational Dynamics: Beyond the Double Helix
A. Non-B DNA Structures
DNA’s canonical B-form helix is frequently disrupted by alternative secondary structures that act as regulatory switches:
- G-Quadruplexes (G4): Formed in guanine-rich regions (e.g., promoters, telomeres), these four-stranded structures recruit transcription factors (TFs) or block RNA polymerase (RNAP) progression. For example, G4 in the MYC oncogene promoter suppresses transcription unless resolved by helicases .
- Triplex DNA (H-DNA): Pyrimidine-rich sequences fold into triple-helix structures, sterically hindering TF binding. Synthetic triplex-forming oligonucleotides (TFOs) can silence genes by stabilizing these conformations .
- Z-DNA: Left-handed helices in CG-rich regions associate with transcriptionally active chromatin, recruiting ADAR1 to regulate RNA editing .
Suggested Figure: Molecular models of B-DNA, G4, and triplex DNA, highlighting structural divergence.
B. Supercoiling and Topological Stress
DNA supercoiling—induced by transcription or replication—alters torsional stress and nucleosome positioning:
- Negative Supercoiling: Unwinds DNA, facilitating RNAP binding and initiation. In E. coli, supercoiling-dependent R-loop formation enhances transcription of stress-response genes .
- Positive Supercoiling: Compacts chromatin, repressing transcription. Topoisomerases (e.g., Topo II) relieve torsional strain to maintain genomic stability .
3. Chromatin Architecture: The 3D Regulatory Landscape
A. Nucleosome Positioning and Histone Modifications
- Nucleosome Phasing: Tightly packed nucleosomes occlude TF binding sites, while nucleosome-free regions (NFRs) expose promoters. Histone acetyltransferases (HATs) loosen chromatin by acetylating lysine residues, enabling transcriptional activation .
- DNA Methylation: CpG island methylation recruits methyl-binding proteins (e.g., MeCP2), compressing chromatin into transcriptionally inert heterochromatin. Hypomethylation at enhancers permits TF access (e.g., OCT4 in pluripotency) .
Suggested Figure: Chromatin states (euchromatin vs. heterochromatin) with labeled histone modifications.
B. Higher-Order 3D Genome Organization
- Chromatin Looping: CTCF/cohesin-mediated loops juxtapose distal enhancers with target promoters. The β-globin locus control region (LCR) loops 50 kb to activate globin transcription .
- Topologically Associating Domains (TADs): Insulated neighborhoods restrict enhancer-promoter interactions. TAD disruptions in cancer cells cause oncogene activation (e.g., MYC in lymphoma) .
- Nuclear Compartmentalization: Active genes localize to transcription hubs near nuclear speckles, while silenced regions cluster at the lamina .
Suggested Figure: 3D genome model showing TADs, loops, and nuclear compartments.
4. Epigenetic Memory and Structural Inheritance
A. DNA Methylation and Heritable Silencing
Parental methylation patterns are propagated during replication:
- Maintenance Methylases (DNMT1): Copy methylation marks to daughter strands, silencing imprinted genes (e.g., IGF2) .
- Transgenerational Epigenetics: Linaria vulgaris flower morphology is inherited via Lcyc gene methylation, bypassing sequence changes .
B. Phase-Separated Condensates
Liquid-liquid phase separation drives the formation of transcriptional hubs:
- Mediator Complex: Condenses with RNAP II at super-enhancers, amplifying oncogene expression in cancer .
- Heterochromatin Protein 1 (HP1): Forms repressive droplets via hydrophobic interactions, spreading H3K9me3 marks .
5. Structural Disruptions in Disease
A. Cancer
- Genome Instability: R-loops and G4 persistence cause replication stress, fueling mutations in BRCA1-deficient cancers .
- Chromatin Remodelers: Mutations in ARID1A (SWI/SNF complex) disrupt enhancer accessibility, promoting metastasis .
B. Neurodegeneration
- Tauopathy: Dysregulated Pol II activity in Alzheimer’s correlates with aberrant CTD phosphorylation and splicing errors .
- Fragile X Syndrome: CGG repeat expansions form stable G4 structures, silencing FMR1 and impairing synaptic plasticity .
6. Technological Innovations Exploiting DNA Structure
A. CRISPR-Based Diagnostics
- SHERLOCK: Uses Cas13a’s collateral RNA cleavage activated by DNA G4 sensors for pathogen detection .
B. Synthetic Biology
- Orthogonal RNAPs: Engineered T7 RNAP variants with altered promoter specificity enable multiplexed gene circuits .
- DNA Origami: Scaffolded G4 nanostructures act as drug carriers targeting telomerase in cancer .
Suggested Figure: CRISPR-Cas9 system guided by DNA structure-sensitive guide RNAs.
7. Future Frontiers
- 4D Nucleomics: Time-resolved cryo-ET captures chromatin dynamics during differentiation .
- AI-Driven Design: Deep learning predicts G4 formation (e.g., G4Boost) and optimizes epigenetic editors .
- Synthetic Chromatin: Engineered histones with non-natural modifications program gene expression in synthetic genomes .
Data Source: Publicly available references.
Contact: chuanchuan810@gmail.com
