Delivergene.com
1. Core Functional Framework: Overcoming Biological Barriers
Gene delivery systems function as molecular transporters engineered to navigate biological obstacles and deliver therapeutic nucleic acids (DNA, RNA, CRISPR components) to target cells. The workflow comprises three critical phases:
A. Vector-Cargo Complexation
- Payload Packaging:
- Viral systems: Therapeutic genes replace viral genomes in capsids (AAV/Lentivirus)
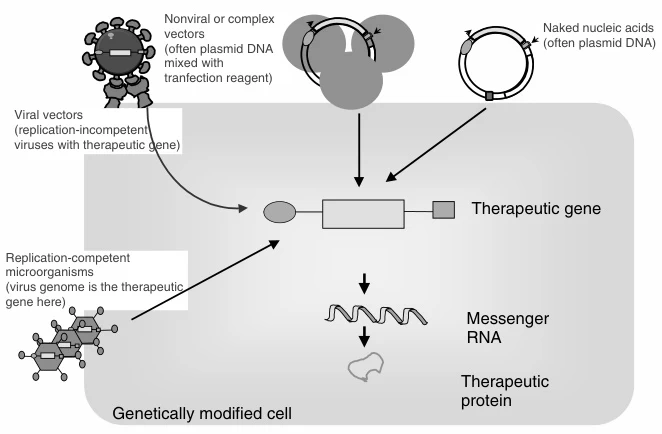
- Non-viral systems: Cationic polymers (e.g., PEI) or lipids electrostatically condense nucleic acids into nanoparticles
- Viral systems: Therapeutic genes replace viral genomes in capsids (AAV/Lentivirus)
- Surface Functionalization: Ligands (e.g., GalNAc, antibodies) enable cell-specific targeting
B. Cellular Entry & Intracellular Trafficking
| Mechanism | Vector Type | Process |
|---|---|---|
| Receptor-Mediated Uptake | Viral/Non-viral | Ligand-receptor binding triggers endocytosis |
| Membrane Fusion | Viral (Adenovirus) | Viral proteins disrupt endosomal membranes |
| Proton Sponge Effect | Polymeric (PEI) | Endosome buffering → osmotic rupture |
C. Nuclear Delivery & Payload Release
- Endosomal Escape: pH-sensitive lipids/polymers destabilize endosomes
- Nuclear Translocation: Viral capsid proteins exploit nuclear import machinery
- Genome Integration/Expression:
- Episomal expression: AAV genomes persist as circular DNA
- Transient expression: mRNA translated in cytoplasm
Suggested Figure 1: Gene Delivery Workflow
[Illustration:
- Left: Vector-DNA complex formation (viral capsid/LNP)
- Center: Cellular uptake via endocytosis (clathrin-coated vesicle)
- Right: Endosomal escape and nuclear entry (viral capsid disassembly)
(Color code: Nucleic acid = blue, Vector = gold, Receptor = purple)]
2. Viral Vector Mechanisms: Harnessing Natural Infectivity
A. Adeno-Associated Virus (AAV)
- Tropism: Serotype-specific targeting (e.g., AAV9 crosses blood-brain barrier)
- Payload Delivery:
- Receptor binding (e.g., HSPG) → Clathrin-mediated endocytosis
- Endosomal acidification → Capsid conformational change
- Nuclear pore transit → Single-stranded DNA release
Suggested Figure 2: AAV Transduction Pathway
[Illustration:
- Step 1: Heparan sulfate proteoglycan (HSPG) binding
- Step 2: Endosomal escape via acidic pH-triggered capsid shift
- Step 3: Nuclear import via nuclear localization signals (NLS)]
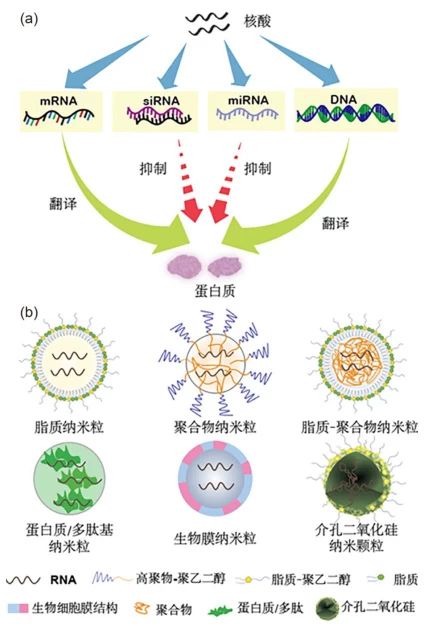
3. Non-Viral Systems: Synthetic Engineering Strategies
A. Lipid Nanoparticles (LNPs)
- Structure: Ionizable lipids + PEG-lipids + cholesterol + nucleic acids
- Key Mechanisms:
- pH-dependent charge switching: Neutral at blood pH → cationic in endosomes
- Endosomal disruption: Lipid fusion with endosomal membranes
B. Polymeric Vectors (e.g., PEI)
- “Proton Sponge” Effect:
- Tertiary amines buffer endosomal pH → Chloride influx → osmotic swelling → endosomal rupture
- Self-Assembly: Branched PEI electrostatically compacts DNA into polyplexes (20-200 nm)
Suggested Figure 3: Polymeric Vector Action
[Illustration:
- Top: PEI-DNA polyplex formation
- Bottom: Proton sponge mechanism showing endosomal rupture (H⁺ influx → osmotic swelling)]
4. Advanced Delivery Technologies
A. Hybrid Systems (e.g., ENVLPE)
- Design: Virus-like particles (VLPs) + synthetic lipids
- Advantages:
- Viral-level efficiency without immunogenicity
- CRISPR cargo protection during intracellular transit
B. Stimuli-Responsive Nanocarriers
| Stimulus | Response Mechanism | Application |
|---|---|---|
| pH Change | Charge reversal → membrane fusion | Tumor microenvironment |
| Redox Gradient | Disulfide bond cleavage → payload release | Intracellular delivery |
5. Tissue-Specific Delivery Engineering
A. Active Targeting
- Ligand Conjugation:
- GalNAc → hepatocyte-specific uptake via ASGPR
- RGD peptides → tumor neovasculature targeting
B. Physical Targeting
- Localized Delivery:
- Intrathecal injection for CNS disorders
- Electroporation-enhanced muscle transfection
Conclusion: Precision Navigation for Genetic Medicine
Gene delivery systems solve three fundamental biological challenges:
- Payload Protection: Shielding nucleic acids from nucleases/degradation
- Barrier Penetration: Crossing cellular membranes and compartments
- Spatiotemporal Control: Ensuring precise release at target sites
While viral vectors leverage evolved infection pathways, non-viral systems employ engineered biomaterials for customizable delivery. Emerging technologies like ENVLPE and stimulus-responsive nanoparticles are bridging efficiency and safety gaps, accelerating therapies for genetic disorders, cancer, and regenerative medicine.
Data sourced from publicly available references. For collaboration or domain inquiries, contact: chuanchuan810@gmail.com.

