Comparative Stability of DNA and RNA: Molecular Determinants and Biological Implications
An Integrated Analysis with Structural Visualizations
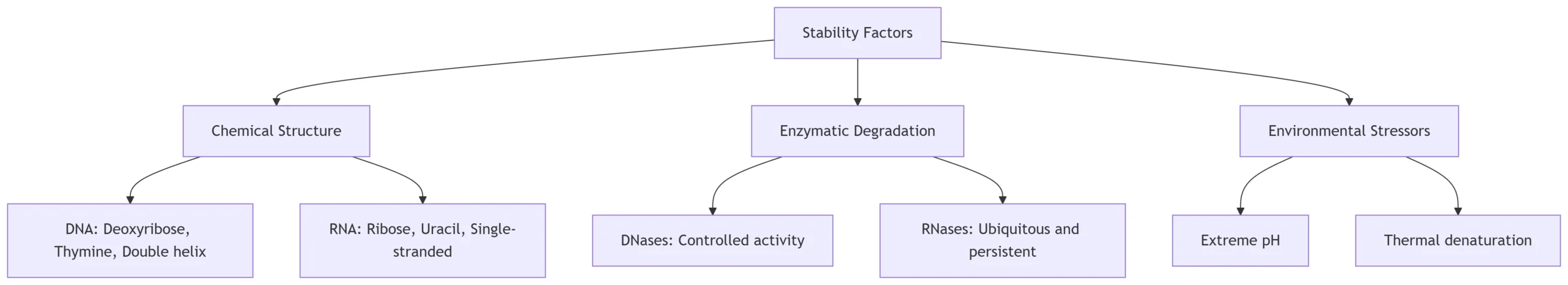
1. Chemical Stability Determinants
DNA Stability Factors:
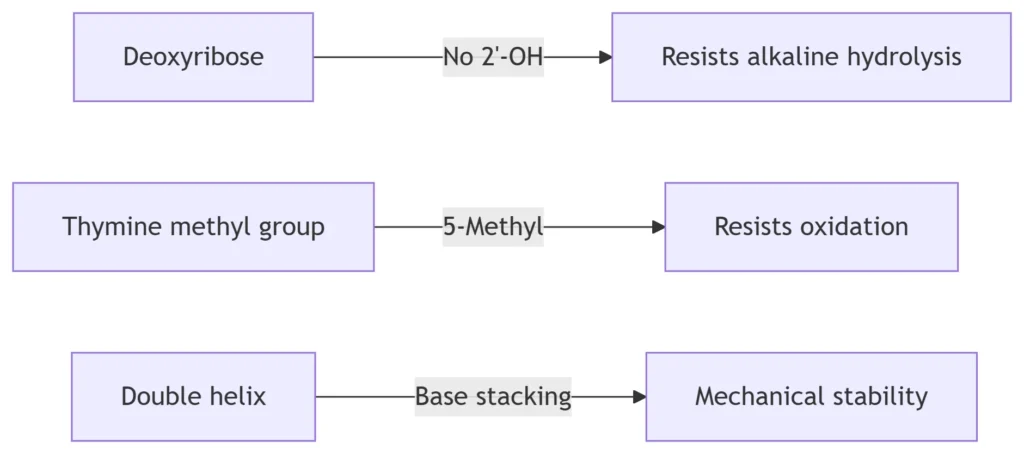
-
Backbone Integrity:
-
C-H bonds in deoxyribose resist hydrolysis (half-life ≈ 30,000 years at 25°C)
-
Methyl group on thymine prevents deamination-induced mutations
-
RNA Vulnerability Hotspots:
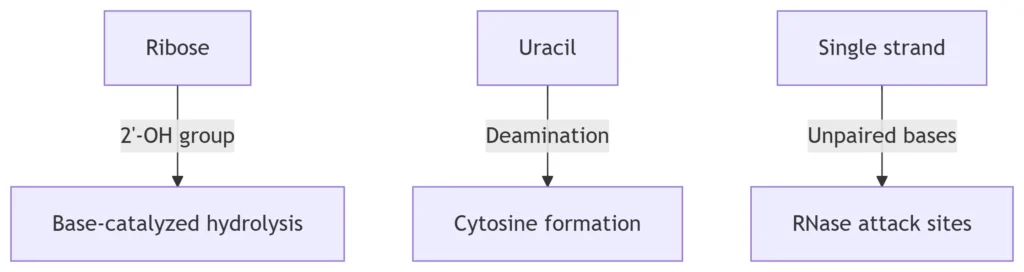
-
Hydrolysis Mechanism:
-
2′-OH group nucleophilically attacks phosphodiester bonds → RNA cleavage
-
Rate increases 100-fold per pH unit above neutrality
-
2. Quantitative Stability Metrics
| Parameter | DNA | RNA |
|---|---|---|
| Alkaline Hydrolysis | Resistant (pH 13, >24 hrs) | Complete degradation (pH 8, 5 min) |
| Thermal Denaturation | T<sub>m</sub> = 85-95°C (GC-rich) | T<sub>m</sub> = 65-75°C (helical regions) |
| Half-life (37°C) | ~200,000 years | <10 minutes (cytoplasm) |
| Deamination Rate | 1/10<sup>7</sup> bases/day | 1/10<sup>4</sup> bases/day |
| Oxidative Damage | 8-oxo-dG lesions (1/10<sup>6</sup> bases) | Fragmentation (no repair systems) |
3. Biological Stabilization Mechanisms
DNA Protection Systems:
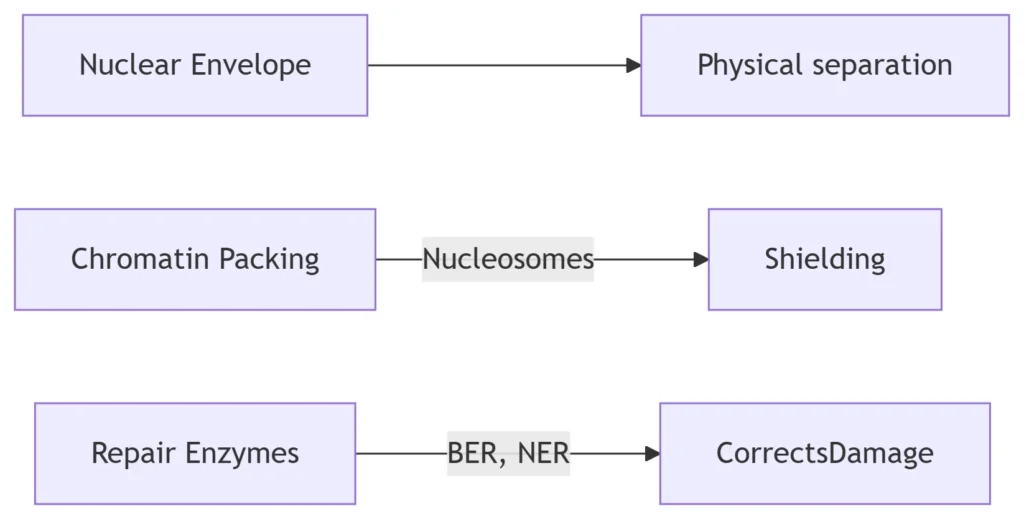
-
Key Proteins:
-
PARP1: Detects DNA breaks
-
XPC: Nucleotide excision repair
-
RNA Surveillance Pathways:
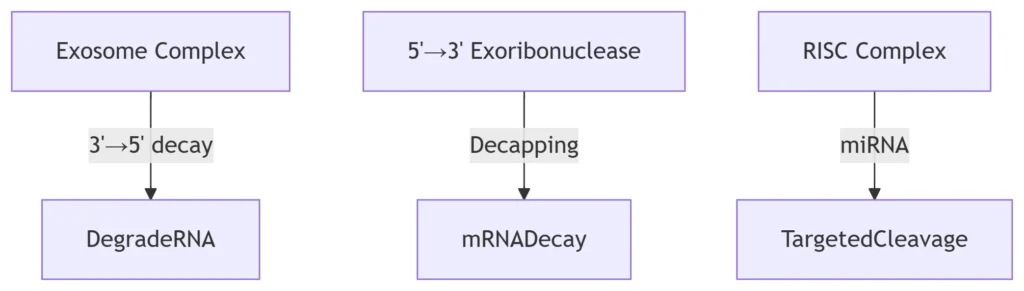
-
Half-life Regulation:
-
Bacterial mRNA: 1-5 minutes
-
Eukaryotic mRNA: 30 min to 24 hours (e.g., β-globin mRNA)
-
4. Environmental Stress Impact
| Condition | DNA Structural Effect | RNA Structural Effect |
|---|---|---|
| High Temperature | Denaturation (reversible above T<sub>m</sub>) | Irreversible unfolding and cleavage |
| UV Radiation | Pyrimidine dimers (260 nm peak) | Chain scission (no photorepair) |
| Acidic pH | Depurination (pH <3) | Rapid phosphodiester hydrolysis |
| Oxidants | 8-oxoguanine lesions | Base modification and fragmentation |
5. Functional Consequences of Stability Differences
DNA Stability Advantages:
RNA Lability Benefits:
6. Technological Applications
Exploiting DNA Stability:
-
PCR: Thermal cycling without backbone degradation
-
DNA Data Storage: 215 PB/g density (Microsoft Project Silica)
-
Ancient DNA Analysis: Sequencing 700,000-year-old fossils
Harnessing RNA Lability:
-
mRNA Vaccines: Transient expression with no genomic integration
-
RNAi Therapeutics: Temporary gene silencing (e.g., Patisiran)
-
Biosensors: Riboswitches with conformation-dependent degradation
Stability Optimization Strategies
| Molecule | Stabilization Method | Application Example |
|---|---|---|
| DNA | Cryopreservation (-80°C) | Biobanking |
| EDTA chelation | PCR reagent formulation | |
| RNA | RNase inhibitors (e.g., SUPERase-In™) | Single-cell RNA sequencing |
| Chemical modification (2′-F, 2′-OMe) | Therapeutic mRNAs |
Conclusion
DNA’s exceptional chemical stability—derived from its deoxyribose backbone, thymine methylation, and double-helical structure—enables reliable genetic information storage across generations. Conversely, RNA’s inherent controlled lability, caused by its ribose 2′-OH group and single-stranded conformation, facilitates rapid response to cellular needs through transient gene expression and regulatory functions. This stability dichotomy is biologically optimized: DNA preserves genetic fidelity while RNA enables adaptive plasticity. Modern biotechnology strategically exploits these properties—leveraging DNA’s durability for data storage and diagnostics, while utilizing RNA’s transience for safe therapeutic interventions.
Data sourced from public references including:
-
Lindahl T. Instability and decay of the primary structure of DNA (Nature, 1993)
-
Draper D.E. RNA folding: thermodynamic and molecular descriptions (Annu Rev Biophys, 2008)
-
NIST Nucleic Acid Stability Database
For research collaboration or content inquiries: chuanchuan810@gmail.com
