
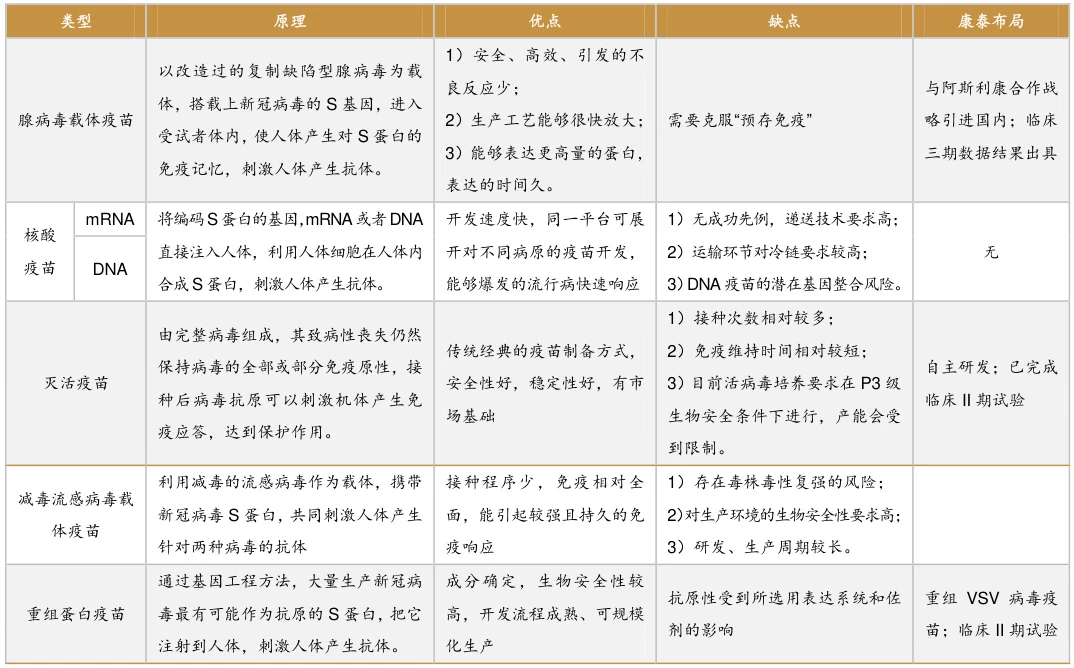
Comparative Analysis of Vaccine Vector Technologies
Vaccine vectors, as a cornerstone of modern vaccinology, are categorized into viral vectors, bacterial vectors, nucleic acid vectors (DNA/mRNA), and synthetic biology vectors. Below is a comprehensive comparison of their advantages, limitations, and applications, optimized for readability and technical depth.
I. Viral Vector Vaccines: Balancing Immunogenicity and Pre-existing Immunity
1. Adenoviral Vectors
- Strengths:
- High infectivity: Single-dose activation of T-cell immunity (e.g., ChAdOx1’s 70% efficacy against COVID-19) .
- Pre-existing immunity evasion: Chimpanzee-derived adenoviruses (e.g., ChAdOx1) avoid neutralization by human antibodies.
- Mucosal delivery: Aerosolized formats (e.g., CanSino’s inhaled vaccine) enhance local immunity.
- Weaknesses:
- Common serotypes (e.g., Ad5) suffer 30–50% efficacy loss due to pre-existing antibodies .
- Theoretical risk of genome integration and carcinogenicity.
- High production costs (HEK293 cell-based systems).
2. Poxvirus Vectors
- Strengths:
- Large genome capacity (>25 kb) for multivalent antigen insertion (e.g., HPV vaccines) .
- Proven safety profile (Modified Vaccinia Ankara, MVA).
- Weaknesses:
- Structural complexity hampers high-throughput development (e.g., 10-year HIV vaccine R&D cycles).
- Systemic rash and fever in some recipients.
3. Vesicular Stomatitis Virus (VSV) & Newcastle Disease Virus (NDV)
- Strengths:
- VSV: Rapid replication enables 97.5% Ebola vaccine efficacy (Ervebo®) .
- NDV: Avian origin avoids human pre-immunity, ideal for zoonotic pathogens.
- Weaknesses:
- VSV: Neurotoxicity requires strict dose control.
- NDV: Low replication efficiency in mammals limits broad use.
II. Bacterial Vector Vaccines: Mucosal Immunity vs. Safety
1. Attenuated Salmonella
- Strengths:
- Oral delivery: Activates gut mucosal immunity (cholera/typhoid prevention).
- Low cost: Production at 1/10 the cost of viral vectors .
- Weaknesses:
- Residual virulence risks (e.g., bacteremia in immunocompromised populations).
- Persistent infection in vulnerable groups.
2. Listeria monocytogenes
- Strengths:
- Dual MHC-I/II antigen presentation: Prolongs survival in tumor-bearing mice (2× extension) .
- Placental barrier penetration for maternal-fetal vaccines.
- Weaknesses:
- Ultra-cold storage (-80°C) triples logistics costs vs. mRNA vaccines.
- Residual toxicity causing febrile gastroenteritis.
III. Nucleic Acid Vectors: Programmability vs. Delivery Challenges
1. mRNA Vaccines
- Strengths:
- Rapid design: 63-day COVID-19 vaccine development (e.g., Moderna mRNA-1273) .
Vaccine Vector - Non-integrating: Safer than DNA vectors; LNPs target lymph nodes (70% biodistribution).
- Rapid design: 63-day COVID-19 vaccine development (e.g., Moderna mRNA-1273) .
- Weaknesses:
- Ultra-cold chain costs (-70°C storage) exceed inactivated vaccines by 5×.
- Nucleoside modifications may disrupt innate immunity (e.g., IFN-α dysregulation).
2. DNA Vaccines
- Strengths:
- Thermostability: 6-month shelf life at ambient temperatures (ideal for tropical regions).
- Long-term protection: Rabies vaccines provide >5-year immunity .
- Weaknesses:
- <0.1% nuclear delivery efficiency; electroporation devices cost ~$500k.
- Plasmid integration risk (1 in 10^6 doses).
IV. Synthetic Biology Vectors: Cutting-edge Innovation
1. Engineered Synthetic Viruses
- Strengths:
- Minimal genomes: Yeast-based systems (90% non-essential gene deletion) reduce off-target effects.
- High payloads: 3DNA® delivers 50+ kb gene clusters (Duchenne muscular dystrophy therapy).
- Weaknesses:
- $100M+ facility investments; only 3 candidates in Phase II trials globally.
2. Plant Virus/Phage Vectors
- Strengths:
- Low-cost production: Tobacco mosaic virus (TMV) at 1/100 mammalian cell costs .
- Gut microbiome targeting: Phage vectors for oral vaccine development.
- Low-cost production: Tobacco mosaic virus (TMV) at 1/100 mammalian cell costs .
- Weaknesses:
- Plant glycosylation triggers human allergies.
- <5% phage survival in gastric acid without encapsulation.
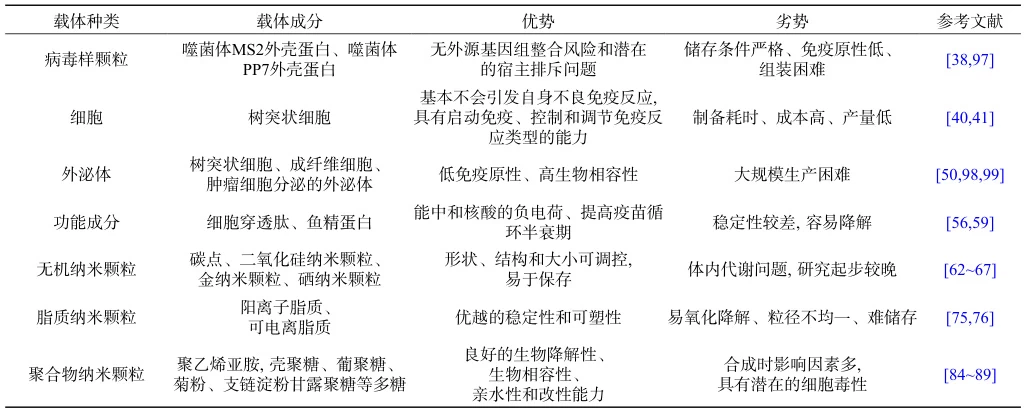
V. Four-Dimensional Evaluation Framework
| Criteria | Viral Vectors | Bacterial Vectors | mRNA Vaccines | Synthetic Vectors |
|---|---|---|---|---|
| Immunogenicity | High (Cell + Humoral) | Moderate (Mucosal) | High (Dual) | Low (Early-stage) |
| Development Speed | Slow (12–24 months) | Moderate (6–12 months) | Rapid (<6 months) | Very Slow (>36 months) |
| Production Cost | Moderate ($10–20/dose) | Low ($1–5/dose) | High ($20–30/dose) | Very High ($100+/dose) |
| Thermostability | Moderate (2–8°C) | Cold chain required | Ultra-cold (-70°C) | High (Ambient) |
VI. Future Innovations
- Pre-existing Immunity Solutions:
- Adenovirus “cocktail” strategies (e.g., Sputnik V’s Ad26/Ad5 mix) boost efficacy to 91% .
- PEG-coated nanoparticles evade antibody recognition.
- Adenovirus “cocktail” strategies (e.g., Sputnik V’s Ad26/Ad5 mix) boost efficacy to 91% .
- Delivery Breakthroughs:
- Hybrid lipid-viral vectors (e.g., Moderna’s LVV) combine LNP targeting and viral efficiency.
- Ultrasound microbubbles enhance DNA vaccine delivery to 30% efficiency.
- AI-Driven Optimization:
- AlphaFold2 predicts antigen-vector binding sites, slashing MVA development by 60% .
- Generative AI designs non-natural nucleotides for 72-hour mRNA stability.
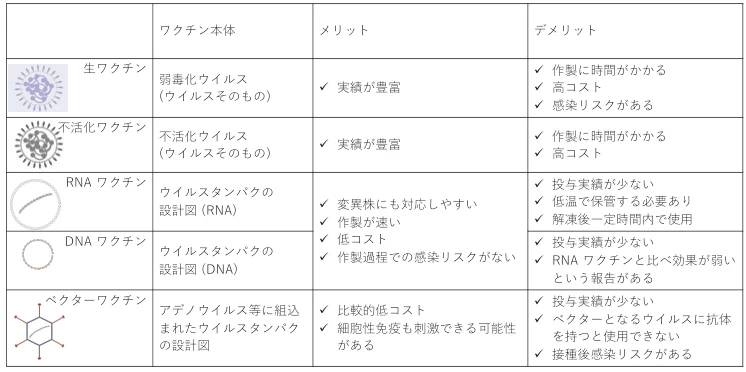
Representative Products Comparison
| Vector Type | Example | Key Advantage | Major Limitation | Application |
|---|---|---|---|---|
| Adenovirus | ChAdOx1 (Oxford) | Single-dose T-cell activation | Pre-existing immunity, integration risks | Pandemic response (e.g., COVID-19) |
| VSV | Ervebo® (Merck) | 97.5% efficacy against Ebola | Neurotoxicity concerns | High-mortality outbreaks |
| Salmonella | ADXS11-001 | Oral delivery, $5/dose cost | Immunocompromised infection risks | Enteric disease prevention |
| mRNA-LNP | BNT162b2 (Pfizer) | 5-day variant adaptation | Ultra-cold chain costs | Rapid pandemic countermeasures |
| Synthetic Yeast | Artemisinin strain | Minimal genome, biosafety | $100M+ facility requirements | Long-term vaccines/gene therapy |
Data sourced from public references. For collaborations or domain inquiries, contact: chuanchuan810@gmail.com.

The Future of Novel Technologies in Vaccine Vector Development
Vaccine vectors, as a cornerstone of modern vaccine development, are undergoing transformative advancements through innovations in virology, nucleic acid engineering, nanomaterials, and artificial intelligence. These breakthroughs are ushering in an era of precision, modularity, and programmability. Below is a comprehensive analysis of emerging trends across technological innovation, expanded applications, and global scalability.
I. Multidimensional Optimization of Viral Vector Technologies
1. Adenoviral Vectors: Overcoming Pre-existing Immunity and Delivery Barriers
Heterologous Prime-Boost Strategies: The Sputnik V vaccine (Ad26/Ad5 hybrid) achieved 91% efficacy by alternating serotypes to evade pre-existing immunity .
Non-humanized Design: ChAdOx1, a chimpanzee adenovirus, avoids human antibody neutralization, enabling single-dose T-cell activation (70% COVID-19 efficacy) .
Mucosal Delivery: CanSino’s inhaled adenovirus vaccine elevates respiratory IgA levels threefold compared to intramuscular administration .
2. Poxvirus and AAV Vectors: Balancing Capacity and Safety
Multipathogen Protection: Poxvirus vectors (>25 kb capacity) co-express HIV envelope and influenza hemagglutinin antigens .
Low Immunogenicity: AAV vectors induce strong antigen-specific responses with minimal capsid reactivity, ideal for chronic diseases like HIV .
3. Avian-derived Vectors: Cross-species Potential
Newcastle disease virus (NDV), with avian-specific tropism, bypasses human pre-immunity and is being tested for H5N1 and Ebola vaccines .
II. mRNA Technology Revolution: From Pandemics to Personalized Medicine
1. Delivery System Enhancements
LNP Optimization: Cationic lipids (e.g., SM-102) increase lymph node targeting to 70% while reducing hepatotoxicity .
Self-amplifying mRNA (saRNA): RNA replicons extend antigen expression to 72 hours, cutting dose requirements by 90% .
2. AI-driven Design
Antigen Prediction: AlphaFold2 optimizes mRNA sequences for epitope conformation, shortening development cycles by 60% .
Synthetic Nucleotides: AI-designed methylation patterns reduce TLR-mediated inflammatory responses .
3. Therapeutic Expansion
Neoantigen Vaccines: BioNTech’s iNeST platform extends melanoma progression-free survival by 4.6 months via personalized mutations .
Protein Replacement: mRNA-encoded Factor VIII sustains expression >28 days in hemophilia models .
III. Next-generation Delivery Systems: Nanotechnology and Biomimetics
1. Multifunctional Nanoparticles
Antigen-Adjuvant Co-delivery: Silica nanoparticles loaded with influenza HA and CpG boost antibody titers eightfold in mice .
Stimuli-responsive Release: Photothermal platinum nanoparticles trigger antigen release in tumors, synergizing with PD-1 inhibitors .
2. Biomimetic Carriers
Virus-like Particles (VLPs): SARS-CoV-2 VLPs mimic native virions, eliciting neutralizing antibodies 3–5× higher than inactivated vaccines .
Bacterial Membrane Coating: E. coli outer membrane vesicles (OMVs) deliver mRNA, enhancing Th1 responses for tuberculosis vaccines .
IV. Synthetic Biology: Modular and Scalable Platforms
1. Engineered Synthetic Vectors
Minimal Genomes: Yeast “chassis cells” with 90% non-essential genes removed produce malaria antigens at BSL-1 safety levels .
Non-viral DNA Carriers: 3DNA® delivers 50 kb gene clusters, restoring 30% dystrophin in Duchenne muscular dystrophy models .
2. Low-cost Manufacturing
Plant-based Production: Tobacco mosaic virus (TMV) expresses influenza antigens at $0.1/dose, ideal for low-resource regions .
Continuous Flow Processes: Modular bioreactors automate mRNA production, achieving 1 billion annual doses .
V. Industrialization and Global Health Impact
1. Combating Viral Variants
Broad-spectrum Vaccines: Adenovirus vectors encoding conserved coronavirus S2 and influenza M2e achieve >60% cross-protection in mice against Beta and Omicron .
Rapid Response: mRNA platforms compress vaccine design-to-production timelines to 30 days for pandemic preparedness .
2. Enhancing Accessibility
Thermostable Formulations: DNA vaccines embedded in trehalose-glass matrices withstand 40°C for 6 months (e.g., ZyCoV-D in India) .
Regional Manufacturing: WHO’s African mRNA hubs produce cholera and malaria vaccines at <$2/dose using modular systems . Future Challenges and Breakthrough Directions Safety Monitoring: Genome-wide integration site databases are needed to track adenoviral vector insertion hotspots . Multivalent Vaccines: CRISPR-Cas9 enables insertion of 10+ epitopes into poxvirus vectors for “super-vaccine” development . Barrier Penetration: Engineered AAV9 crosses the blood-brain barrier, achieving protective rabies antibody levels in primates . Conclusion The convergence of novel technologies is transforming vaccine vectors from passive defenses into actively engineered solutions for infectious diseases, cancer, and genetic disorders. Over the next five years, advancements in AI-synthetic biology integration, nanodelivery systems, and decentralized production will solidify vaccine vectors as indispensable tools against pathogens and chronic conditions. Data sourced from public references. For collaborations or domain inquiries,