 1. Core Production Frameworks and Time Determinants
1. Core Production Frameworks and Time Determinants
Synthetic vaccines—encompassing mRNA, synthetic peptides, and virus-like particles (VLPs) —leverage de novo design and cell-free synthesis. Their production cycles vary significantly based on:
- Platform technology (mRNA vs. peptide vs. viral vector)
- Scale (preclinical batches vs. commercial manufacturing)
- Regulatory pathways (standard vs. emergency use)
Key Time Drivers:
| Phase | Duration Range | Critical Factors |
|---|---|---|
| Antigen Synthesis | 1–30 days | Sequence length, cyclization, purity |
| Formulation | 3–14 days | Nanoparticle encapsulation, lyophilization |
| Quality Control (QC) | 14–60 days | Sterility, potency, stability testing |
2. mRNA Vaccine Production: A 40-Day Standard
Stepwise Timeline
- DNA Template Preparation (1–2 days):
- Linearized plasmid DNA encoding antigen is amplified in E. coli bioreactors .
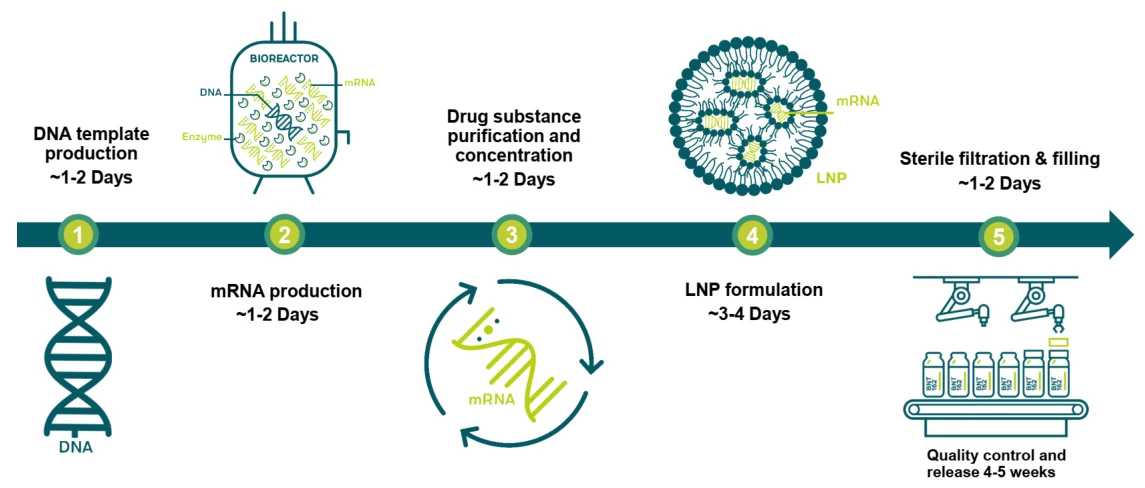
- Linearized plasmid DNA encoding antigen is amplified in E. coli bioreactors .
- mRNA Synthesis (1–2 days):
- In vitro transcription (IVT) with T7 RNA polymerase yields mRNA strands .
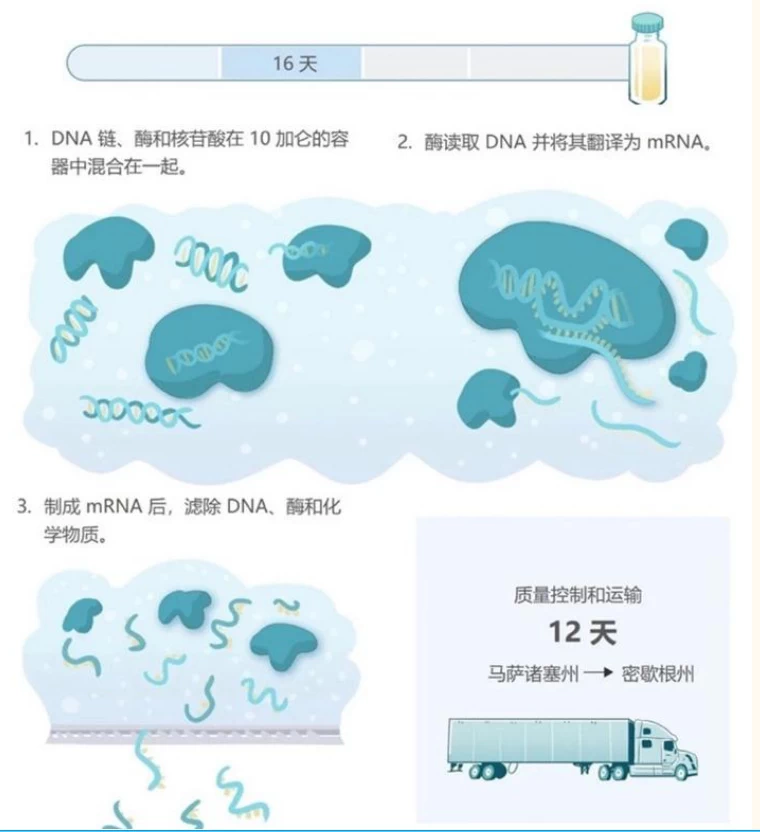
- In vitro transcription (IVT) with T7 RNA polymerase yields mRNA strands .
- Purification & LNP Encapsulation (3–4 days):
- Tangential flow filtration removes impurities; lipid nanoparticles (LNPs) assemble via microfluidics .
- Tangential flow filtration removes impurities; lipid nanoparticles (LNPs) assemble via microfluidics .
- Sterile Filling (1–2 days):
- Vials are filled under cGMP conditions .
- QC and Release (28–35 days):
- Tests include endotoxin, sterility, identity (HPLC), and potency (cell-based assays) .
Total: 34–45 days
Suggested Figure 1: mRNA Vaccine Production Workflow
- DNA Template → IVT → Purification → LNP Formulation → Filling → QC Release.
- (Colors: DNA = blue, mRNA = purple, LNPs = gold, QC tests = red/amber/green)
3. Synthetic Peptide Vaccines: 60–90 Days for Complex Antigens
Critical Phases
- Peptide Synthesis (20–22 days):
- Solid-phase peptide synthesis (SPPS) builds sequences up to 50 aa .
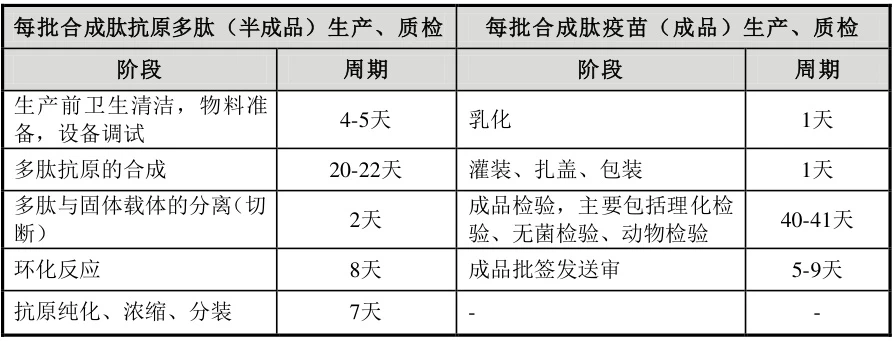
- Solid-phase peptide synthesis (SPPS) builds sequences up to 50 aa .
- Cyclization & Purification (8–10 days):
- Disulfide bonding/HPLC purification achieves >95% purity .
- Conjugation & Emulsification (8–10 days):
- Carrier proteins (e.g., KLH) are linked via SpyTag/SpyCatcher chemistry .
- QC (40–41 days):
- In vivo immunogenicity testing dominates timelines .
Total: 76–83 days
Suggested Figure 2: Peptide Vaccine Manufacturing
- SPPS → Cyclization → Conjugation → Emulsification → QC.
- (Colors: Peptides = teal, carriers = gray, adjuvants = orange)
4. Accelerated Timelines During Pandemics
COVID-19 Case Study
- mRNA Vaccines (Pfizer/Moderna):
- Production compressed to 40 days via parallel processing and regulatory waivers .
- QC overlapped with manufacturing (“rolling review”) .
- Viral Vector Vaccines (AstraZeneca):
- 22-day cell culture + 18-day purification = 40-day total .
Suggested Figure 3: Pandemic Acceleration Strategies
- Timeline comparison: Traditional (12+ months) vs. COVID-19 (40 days).
- Key accelerators: Modular factories, real-time QC, emergency approvals.
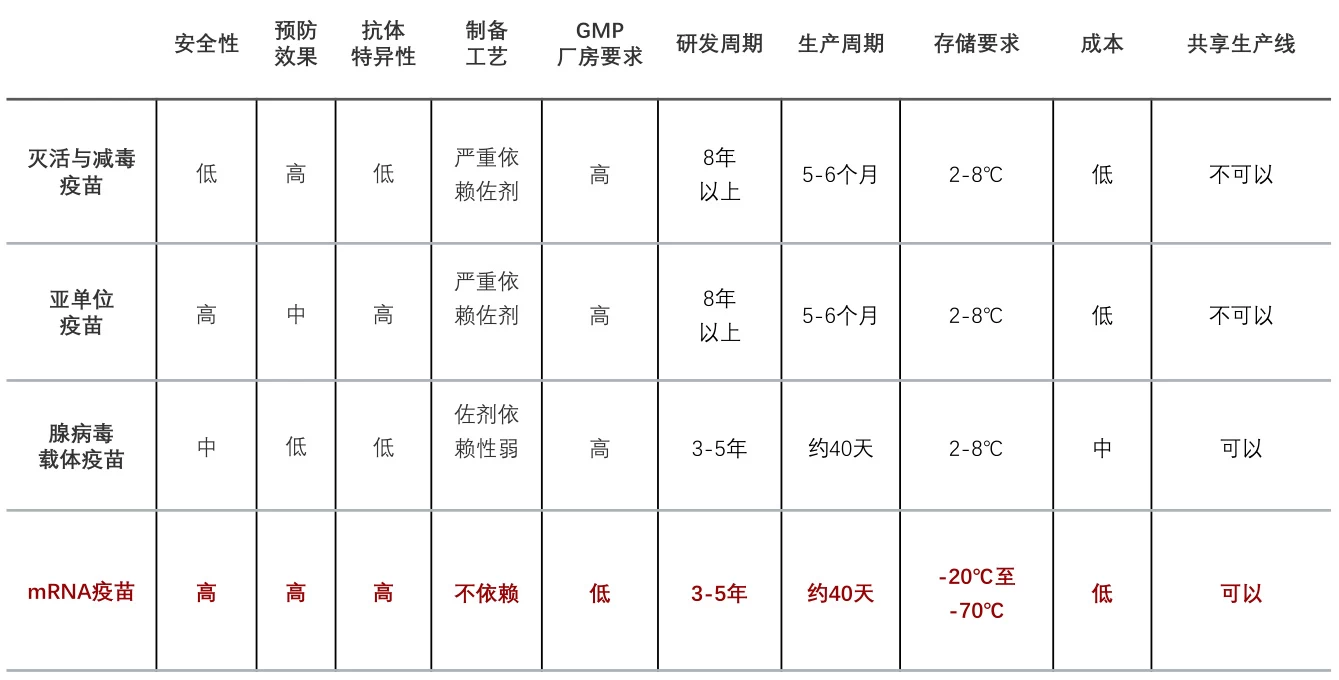
5. Comparison to Traditional Vaccines
| Vaccine Type | Production Time | QC Dominance |
|---|---|---|
| Synthetic mRNA | 34–45 days | 65–70% of cycle |
| Synthetic Peptide | 76–83 days | 50–55% of cycle |
| Inactivated Viral | 5–6 months | 40–50% of cycle |
| Live-Attenuated | 8–12 months | 30–40% of cycle |
QC includes sterility, potency, and stability assays
6. Innovations Reducing Future Timelines
- AI-Driven QC: Machine learning predicts sterility failures, cutting validation by 50% .
- Continuous Manufacturing: Integrated microfluidic systems synthesize and encapsulate mRNA in <7 days .
- Lyophilized Formulations: Eliminate cold chain, reducing stability testing from 6 months to 4 weeks .
Conclusion
Synthetic vaccine production cycles range from 34 days (mRNA) to 83 days (complex peptides), dominated by rigorous QC. mRNA platforms achieve the shortest timelines through:
- Cell-free in vitro transcription
- Modular nanoparticle formulation
- Parallelized quality testing
Pandemic responses proved these can be compressed to 40 days via regulatory agility and process innovation. As AI and continuous manufacturing mature, synthetic platforms will enable <30-day end-to-end production for future outbreaks.
Data Source: Publicly available references.
Contact: chuanchuan810@gmail.com
