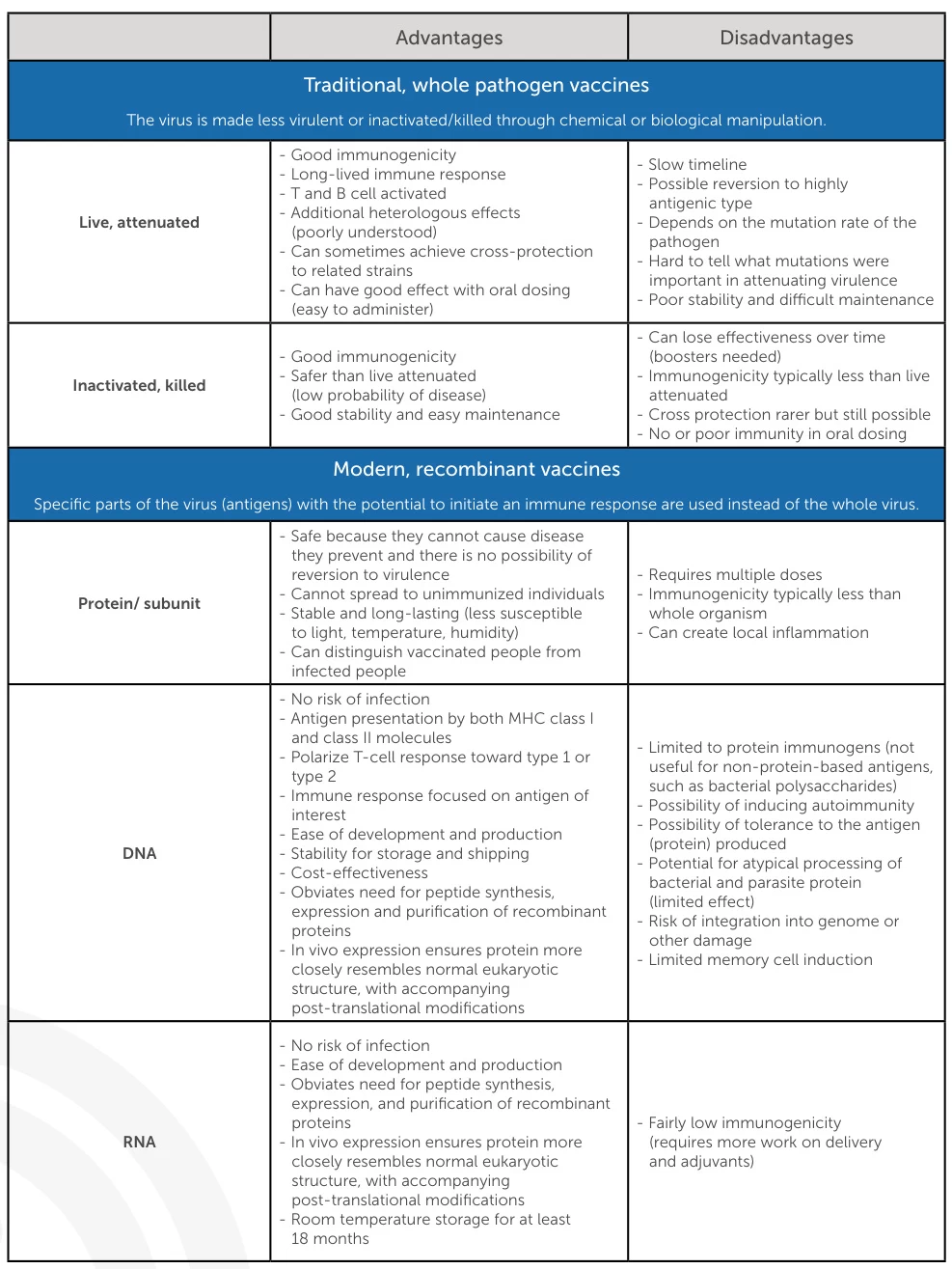
 1. Fundamental Definitions and Core Principles
1. Fundamental Definitions and Core Principles
| Vaccine Type | Definition | Key Components |
|---|---|---|
| Recombinant Vaccines | Engineered by inserting pathogen genes into living vectors (yeast, bacteria, or viral carriers) to express target antigens. Antigens are purified for immunization. | – Viral/bacterial vectors (e.g., adenovirus, Saccharomyces cerevisiae) – Pathogen-derived antigen genes (e.g., hepatitis B surface antigen) |
| Synthetic Vaccines | De novo synthesized using computational design and chemical assembly; no biological components involved. | – Synthetic peptides/nucleotides (e.g., mRNA, DNA) – Artificially constructed antigens (e.g., SpyTag/SpyCatcher assemblies) |
Suggested Figure 1: Molecular Design Contrast
- Left (Recombinant): Adenovirus vector (blue) carrying pathogen gene (red) → antigen expression in host cells.
- Right (Synthetic): Chemically assembled peptide-MHC complex (gold) or mRNA-LNP (purple/gold).
2. Production Technologies and Engineering Platforms
A. Recombinant Vaccine Production
- Gene Cloning: Pathogen antigen gene (e.g., COVID-19 spike protein) is inserted into vector DNA (e.g., yeast plasmid).
- Biological Expression: Vectors are cultured in bioreactors; antigens are harvested and purified.
- Examples:
- Hepatitis B Vaccine: Yeast-expressed HBsAg.
- HPV Vaccine: Virus-like particles (VLPs) from S. cerevisiae.
B. Synthetic Vaccine Production
- Computational Design: Epitopes predicted via AI (e.g., SARS-CoV-2 spike epitopes).
- Chemical Synthesis:
- Peptide-based: Solid-phase peptide synthesis (SPPS).
- Nucleic acid-based: In vitro transcription (mRNA) or gene-synthesized DNA.
- Self-Assembly Systems: SpyTag/SpyCatcher enables plug-and-play multi-antigen complexes.
Suggested Figure 2: Manufacturing Workflows
- Recombinant: Gene insertion → vector amplification → antigen purification.
- Synthetic: In silico epitope design → chemical synthesis → formulation (e.g., LNP encapsulation).
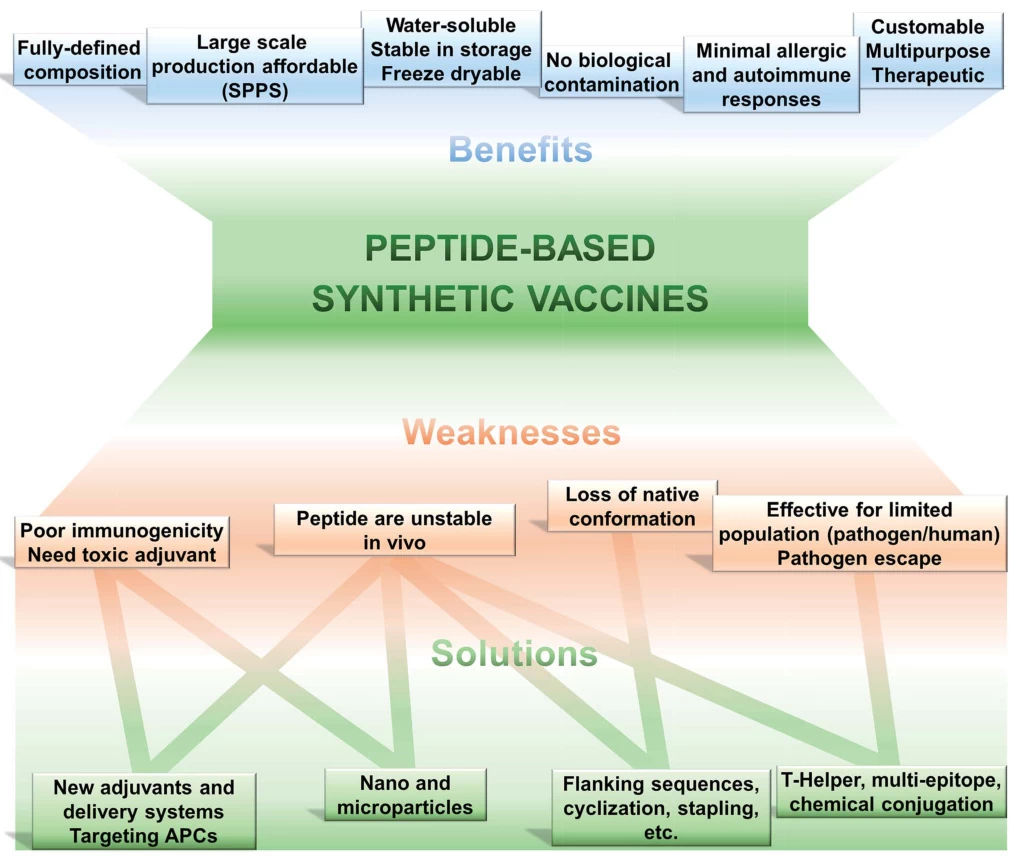
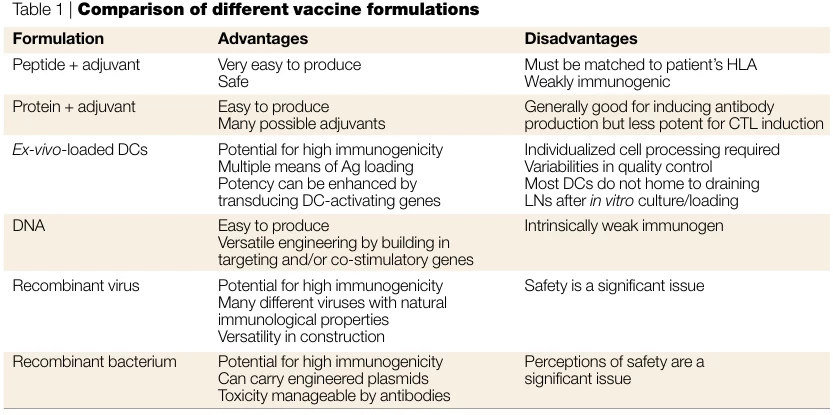
3. Key Advantages and Limitations
| Parameter | Recombinant Vaccines | Synthetic Vaccines |
|---|---|---|
| Safety | Low risk of replication-competent pathogens; no genome integration. | No biological material; avoids contamination risks. |
| Development Speed | 6–12 months (depends on vector optimization). | Weeks to months (e.g., Moderna COVID-19 mRNA vaccine). |
| Immunogenicity | Requires adjuvants (e.g., aluminum salts); mimics native antigen structures. | Poor innate immunogenicity; needs novel adjuvants/nanocarriers. |
| Thermostability | Stable at 2–8°C (e.g., Gardasil). | mRNA requires ultra-cold storage (−20°C to −70°C). |
| Production Scalability | Limited by cell-culture capacity. | Fully synthetic process; no bioreactors needed. |
4. Immunological Mechanisms
A. Recombinant Vaccines
- Antigen Presentation: Vector-infected cells process and display antigens via MHC-I/II, activating CD8+/CD4+ T cells.
- Humoral Response: Purified antigens induce neutralizing antibodies (e.g., anti-HBsAg IgG).
B. Synthetic Vaccines
- Peptide Vaccines: Direct uptake by dendritic cells → MHC-restricted T-cell activation.
- mRNA Vaccines: Host cells translate mRNA into antigens, triggering cytotoxic T cells and antibodies.
Suggested Figure 3: Immune Activation Pathways
- Recombinant: Vector entry → antigen expression → MHC presentation → T/B-cell activation.
- Synthetic: mRNA translation (ribosomes) → antigen degradation → dendritic cell priming.
5. Clinical and Commercial Applications
| Disease Target | Recombinant Vaccine | Synthetic Vaccine |
|---|---|---|
| COVID-19 | CanSinoBio (adenovirus vector). | Moderna (mRNA-LNP), CureVac (RNA). |
| Hepatitis B | Engerix-B (yeast-expressed HBsAg). | Peptide-based candidates (preclinical). |
| Malaria | Salmonella-expressed Plasmodium antigens (preclinical). | Synthetic Plasmodium peptides + nanocarriers. |
| Cancer | HPV VLP vaccines (e.g., Gardasil). | Neoantigen peptide/RNA personalized vaccines. |
6. Future Innovations and Convergence
- Hybrid Designs:
- Recombinant vectors delivering synthetic antigens (e.g., adenovirus-mRNA).
- SpyTag/SpyCatcher-functionalized VLPs.
- AI-Driven Platforms:
- Recombinant: Optimized codon usage for enhanced antigen expression.
- Synthetic: Quantum computing-predicted epitope stability.
- Delivery Breakthroughs:
- Lyophilized mRNA for tropical regions.
- Yeast-based oral recombinant vaccines.
Suggested Figure 4: Next-Gen Vaccine Convergence
Hybrid nanoparticle with recombinant vector core (blue) and synthetic antigen coating (gold).
Conclusion
Recombinant and synthetic vaccines represent distinct technological paradigms:
- Recombinant vaccines leverage biological systems to produce native-like antigens, enabling robust immune responses but constrained by vector biology.
- Synthetic vaccines exploit computational design and chemical synthesis for unprecedented speed and flexibility, though stability and immunogenicity challenges persist.
The future lies in merging these approaches—recombinant platforms delivering synthetic antigens—to create “designer vaccines” against evolving pathogens and cancers.
Data Source: Publicly available references.
Contact: chuanchuan810@gmail.com
