 Introduction
Introduction
RNAScan—a suite of targeted RNA analysis technologies—achieves maximal accuracy through specialized reagents and consumables designed to minimize contamination, stabilize RNA structures, and enhance signal specificity. This article delineates the essential components for optimizing RNAScan workflows, categorized by sample preparation, hybridization, detection, and quality control. Each section integrates proprietary innovations that elevate sensitivity, reduce background noise, and enable multiplexed analysis.
1. Sample Preparation: Ensuring RNA Integrity
A. Tissue Fixation and Sectioning
- Fixatives:
- 10% Neutral Buffered Formalin (NBF) or 4% Paraformaldehyde (PFA) for structural preservation (#user-content-1)(#user-content-5).
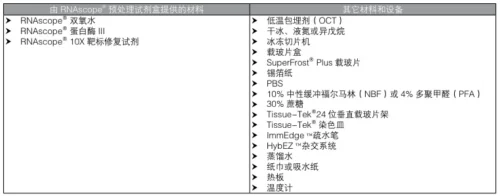
- RNAlater™ for stabilizing RNA in fresh tissues during storage/transport (#user-content-13).
- 10% Neutral Buffered Formalin (NBF) or 4% Paraformaldehyde (PFA) for structural preservation (#user-content-1)(#user-content-5).
- Sectioning Consumables:
- SuperFrost™ Plus Slides: Required to prevent tissue detachment during stringent hybridization (#user-content-5)(#user-content-7).
- Cryoprotectants: 30% Sucrose for OCT-embedded frozen tissues (#user-content-1).
B. RNA Extraction and Purification
- RNA Extraction Kits:
- StarPure RNA Kit (polysaccharide-rich samples) or StarSpin Plant RNA Kit (polyphenol-rich plants) (#user-content-14).
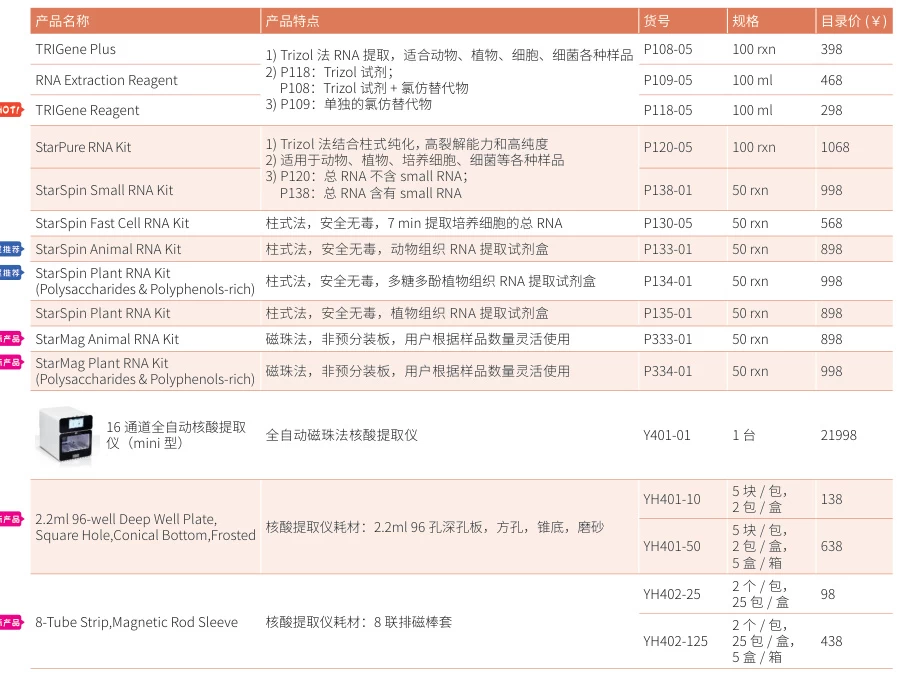
- High Pure FFPE RNA Micro Kit for degraded clinical samples (RIN >8.0) (#user-content-4).
- StarPure RNA Kit (polysaccharide-rich samples) or StarSpin Plant RNA Kit (polyphenol-rich plants) (#user-content-14).
- Nuclease-Free Consumables:
- Axygen RNase-Free Tubes/Tips (1.5 mL tubes: MCT-150-C; tips: 10/200/1000 μL) (#user-content-4)(#user-content-15).
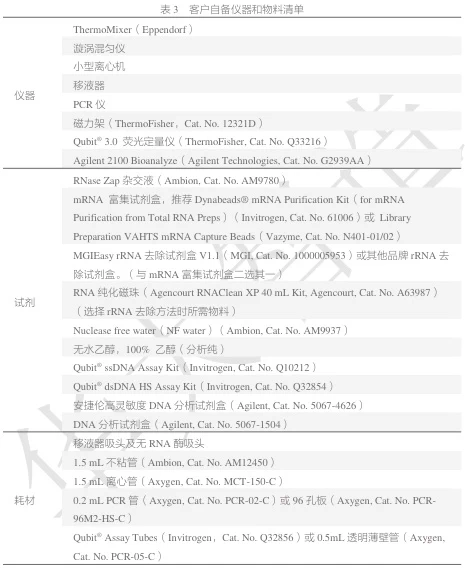
- RNaseZap™ for surface decontamination (#user-content-10)(#user-content-13).
- Axygen RNase-Free Tubes/Tips (1.5 mL tubes: MCT-150-C; tips: 10/200/1000 μL) (#user-content-4)(#user-content-15).
Suggested Figure: Sample Prep Workflow: Tissue → NBF/PFA fixation → OCT embedding → SuperFrost™ Plus sectioning → RNase-free extraction.
2. Hybridization and Target Enrichment
A. Probe Hybridization Systems
- HybEZ™ II Oven: Maintains controlled humidity/temperature for RNAscope assays (#user-content-7).
- ImmEdge™ Hydrophobic Barrier Pen: Confines reagents to tissue sections, preventing cross-contamination (#user-content-1)(#user-content-7).
B. Target-Specific Reagents
- Pretreatment Kits:
- RNAscope® Pretreatment Kit: Includes hydrogen peroxide (H₂O₂), Protease Plus, and Target Retrieval reagents (#user-content-1)(#user-content-8).
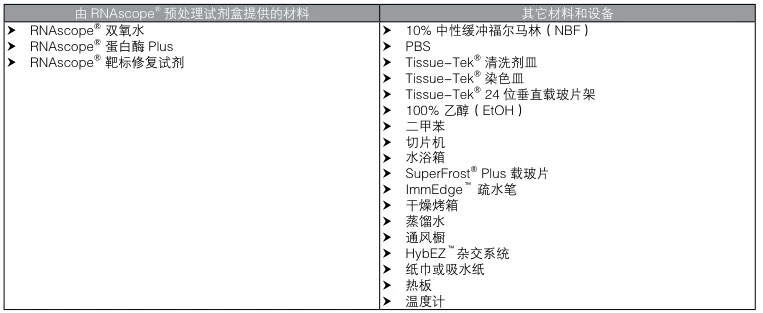
- RNAscope® Pretreatment Kit: Includes hydrogen peroxide (H₂O₂), Protease Plus, and Target Retrieval reagents (#user-content-1)(#user-content-8).
- Hybridization Buffers:
- RNAscope® Wash Buffer (310091) for stringent washes (#user-content-3).
- Deionized Formamide for probe binding optimization (#user-content-11).
Suggested Figure: Hybridization Setup: Tissue section → ImmEdge™ barrier → HybEZ™ II oven → Probe incubation.
3. Signal Amplification and Detection
A. Multiplex Fluorescent Detection
- RNAscope® Multiplex Fluorescent Kit v2 (323110): Includes AMP 1, AMP 2, AMP 3, and protease reagents (#user-content-3).
- Ancillary Kit (323120): Required for 4-plex detection.
- Tyramide Signal Amplification (TSA):
- PerkinElmer TSA/Opal Dyes: e.g., Opal 520, 570, 690 for multiplexed imaging (#user-content-3).
B. Chromogenic Detection
- RNAscope® 2.5 HD Detection Reagents:
- BROWN (322310) or RED (322360) for single-plex assays (#user-content-3).
- Duplex Detection (322500) for two-target co-localization.
Suggested Figure: Signal Amplification: Probe binding → AMP 1-3 cascade → TSA/Opal dye conjugation.
4. Imaging and Mounting
A. Counterstaining and Mounting
- Counterstains:
- Gill’s Hematoxylin I (1:2 dilution) for nuclear staining (#user-content-5).
- Mounting Media:
- VectaMount™ for Brown assays (xylene-free) (#user-content-7).
- EcoMount™ for Red assays (#user-content-5).
B. Advanced Imaging Consumables
- BeyoClick™ EU-488 Kit: Click chemistry reagents for RNA synthesis imaging (#user-content-6).
- Anti-Fade Reagents: e.g., P0126 Mounting Medium to prevent fluorophore quenching (#user-content-11).
5. Quality Control and Quantification
A. RNA Integrity Verification
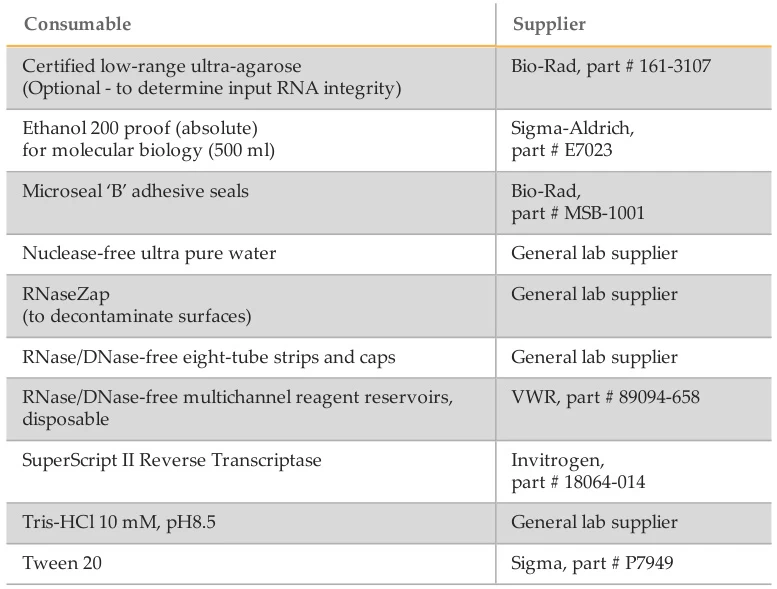
- Agilent 2100 Bioanalyzer:
- RNA 6000 Nano Kit (5067-1511) for total RNA/mRNA QC (#user-content-9)(#user-content-16).
- Small RNA Kit (5067-1548) for microRNA analysis.
- Qubit® 3.0 Fluorometer:
- Qubit® RNA HS Assay Kit for low-concentration RNA (#user-content-15).
B. Library Construction QC
- Agencourt RNAClean XP Beads (A63987) for post-enrichment purification (#user-content-15).
- Agilent High Sensitivity DNA Kit (5067-4626) for NGS library validation (#user-content-15).
Suggested Figure: QC Pipeline: Bioanalyzer electropherogram → Qubit® quantification → Bead purification.
6. Specialized Reagents for Advanced Workflows
| Application | Reagent/Kit | Key Feature |
|---|---|---|
| CRISPR-RNAScan | Cas13 mRNA-LNPs | Tissue-targeted delivery (#user-content-6) |
| Single-Cell RNAScan | Nano-UMI Adapters (20-nt barcodes) | Single-molecule error correction (#user-content-15) |
| R-Loop Profiling | RiboGreen™ RNA Quantitation Reagent | Detects RNA-DNA hybrids (#user-content-12) |
| Fusion Detection | QIAseq Targeted RNAScan Panels | UMI-based error suppression (#user-content-2) |
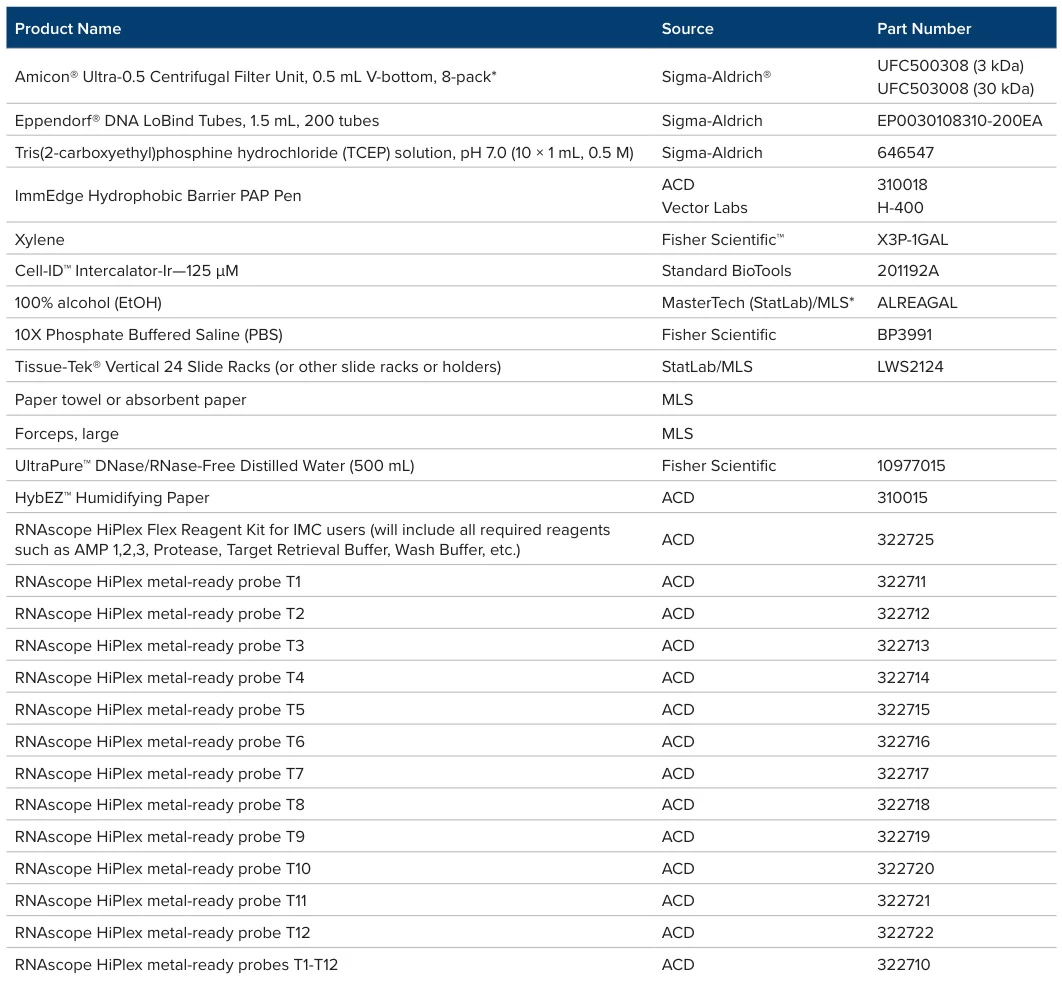
Conclusion
Optimizing RNAScan demands precision-grade reagents and consumables at every stage:
- Sample Prep: SuperFrost™ Plus Slides and RNase-free tips/tubes prevent degradation.
- Hybridization: HybEZ™ II and ImmEdge™ Pen ensure target-specific probe binding.
- Detection: TSA/Opal Dyes enable multiplexed imaging with low background.
- QC: Bioanalyzer/Qubit® systems validate RNA integrity pre/post-analysis.
By integrating these specialized components, RNAScan achieves 0.1% sensitivity for fusion detection, single-cell resolution, and clinical-grade reproducibility.
Data Source: Publicly available references.
Contact: chuanchuan810@gmail.com
